February 4, 2015 report
Why do our photoreceptors respond to light by turning off?
(Medical Xpress)—An enduring neurobiological mystery is why do vertebrate rods and cones shut down their transmitter release in response to a light stimulus. If that particular question is too broad, then consider a slight refinement: why do we use two kinds of hyperpolarizing detectors in our retina while invertebrates like flies use a single depolarizing photoreceptor instead? That might be something we could answer, if only our understanding of invertebrate phototransduction was as complete as that of our own. Fortunately, a theory which ties together some of the absent details has been conveniently supplied in a recent review article in Current Opinion in Neurobiology.
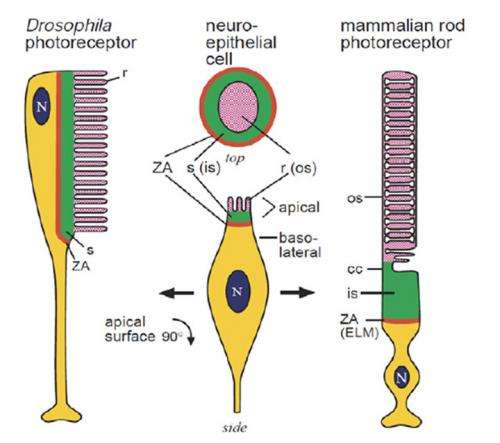
Drosophila use a standard issue rhabdomeric r-opsin invertebrate eye complete with compartmentalized microvillar-style photoreceptors. Behind the scenes, a typical phosphoinositide cascade plays out in these photoreceptors where phospholipase C (PLC) hydrolyzes (PIP2) into diacylglycerol (DAG) and inositol trisphosphate (InsP3). The missing link that is needed, is what is the chemical pathway that then turns on the TRP (transient receptor potential) and TRPL calcium channels?
What authors Roger Hardie and Mikko Juusola think is actually happening here, is that there is no simple chemical pathway that explains all the existing experimental data. Instead, their own experiments suggest that a photomechanical pathway exists where PLC mediates contractions of photoreceptor cells and subsequent activity of mechanosensitive channels. In previous work, they and others used an AFM microscope to measure the time course and sensitivity of the rapid movements within microvilli to various manipulations. They found that the latency of the mechanical response was shorter than the associated electrical response and was synchronized with PIP2 hydrolysis.
As PIP2 is an abundant integral membrane phospholipid, cleavage by PLC, (and the ensuing emigration of bulky and well charged InsP3 headgroups) would create quite a kerfuffle in the membrane. In addition to affecting its thickness, area, volume, and lateral pressure, the asymmetric distribution with respect to inner and outer leaflets would also be expected to induce curvature changes. The experiments also showed, however, that PIP2 alone is not the whole story.
To take a step back for a moment, there is another age old neurobiological question whose explanation could potentially shed as much general evolutionary light as the question of hyperpolarizing transduction. That is, why do these same invertebrates primarily utilize glutamate at their neuromuscular junctions while more civil beings like you and I prefer acetylcholine (Ach)? Although that is a question for another post, suffice it to say that one recent finding that bears on this question is that the lowly protons generated by Ach cleavage turn out to have significant chemical and mechanical effects on the membrane and its localized proteins.
In our recent post on solitons and sonic booms in membranes we also described experiments showing that cleavage products of Ach (specifically the protons), as opposed to classical Ach itself, excite spikes in Chara cells. There is more on the thermodynamic and mechanical effects of protons and pH changes in general to be read there. For the issue at hand, what we neglected to mention above, is that DAG and InsP3 are not the only products evolved by PLC. In fact, the mechanical effect of the depletion of PIP2 in membranes appears to occur only in combination with the protons that also happen to be released after direct PLC action.
Regarding the general question of photoreceptor efficiency, we previously looked at some of the trade-offs made when photoreceptors, bipolar cells, and ganglion cells use spikes as opposed to just graded potentials. While not generally thought of as spatially-extended spiking cells, one study that was looking further into hyperpolarization-activated cation channels noted that primate photoreceptors can nonetheless show spike-like currents in response to termination of a light stimulus.
While it may be a peripheral issue, the mention above of solitons and pressure pulses also points to an often neglected peculiarity regarding this kind of signalling as it relates to spikes or graded potentials. That is, it shouldn't matter so much whether a disturbance is electrically excitatory or inhibitory—if it is sharp enough, it should still lead to a proper flesh and blood mechanical pulse. This same general concept, the sweeping idea of relative or derivative signalling might also be found to be critical to circuit-level operation of the cerebellum where inhibition is often noted to play a critical role in punctuating its primary output.
When I asked Roger Hardie about the polarity of membrane potential changes in our photoreceptors, he offered that while there is no generally accepted explanation for their negative attitude toward light, it is likely easier to signal that a light stimulus is present by depolarization like the fly does. The catch is does easier just mean energetically favorable, and if so how does that encompass the retinal ideals of speed, reliability and adaption to light level? As his paper describes, fly photoreceptors are fast; they respond to single photons 10 to 20 times more rapidly than vertebrate rods, and they can still signal under full sunlight. This fast response has been accounted for, at least in the models described, by bidirectional feedback from calcium channels within the highly compartmentalized microvilli.
Regarding this energy question, Roger pointed to Simon Laughlin et. al.'s ATP neuroaccounting paper which addresses energy consumption as a function of voltages and currents in photoreceptors. They find that in darkness, rods consume around 10^8 ATP per second—roughly similar to Drosophila photoreceptors. They determine that rods are metabolically less costly than cones because cones don't saturate in bright light and they also use more ATP in their downstream signalling pathways.
Although the choice of photoreceptor polarity used by various classes of life (perhaps much like the choice of transmitter at the neuromuscular junction) may never be answered exactly, it will undoubtedly continue to gain better explanations. The energy used in signaling and the rapid adaption of the various pathways that maintain constancy with changing light levels is certainly one productive approach, but probably not the whole story.
The energy needed for the slower scale structural modifications—the physical connectivity modifications that happen in our retina on roughly 10 minute adaption scales, and the even slower circadian conservation measures many creatures employ each sunset—still needs to be better defined to really know the retina's operating principles. Compared to the ATP needed for pumping out sodium and restoring membrane potential, the ATP needed to reconnect with say for example, twenty bipolar cells, may be just as important—but a bit harder to get a handle on.
More information: Phototransduction in Drosophila, Current Opinion in Neurobiology, www.sciencedirect.com/science/ … ii/S0959438815000173
Abstract
Phototransduction in Drosophila's microvillar photoreceptors is mediated by phospholipase C (PLC) resulting in activation of two distinct Ca2+-permeable channels, TRP and TRPL. Here we review recent evidence on the unresolved mechanism of their activation, including the hypothesis that the channels are mechanically activated by physical effects of PIP2 depletion on the membrane, in combination with protons released by PLC. We also review molecularly explicit models indicating how Ca2+-dependent positive and negative feedback along with the ultracompartmentalization provided by the microvillar design can account for the ability of fly photoreceptors to respond to single photons 10–100× more rapidly than vertebrate rods, yet still signal under full sunlight.
© 2015 Medical Xpress