June 19, 2015 feature
Simple hydrogen storage solution is powered by solar energy
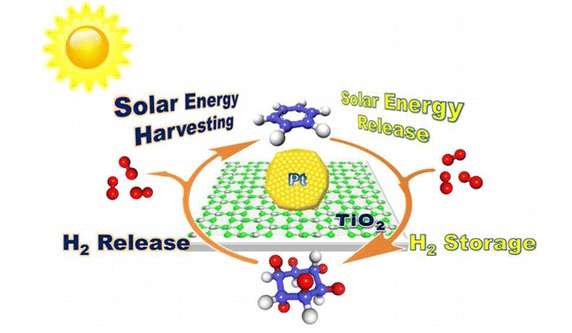
(Phys.org)—By using solar energy to reversibly attach and detach hydrogen atoms on a 6-carbon ring called benzene, scientists have developed a simple and efficient method to store, transport, and release hydrogen potentially on a large scale. The hydrogen storage problem is currently one of the biggest challenges facing the development of hydrogen as a widespread energy carrier, and the researchers hope that the new strategy may lead to a safe and inexpensive solution to this problem.
The scientists, led by Professor Chao-Jun Li and Associate Professor Zetian Mi at McGill University in Montreal, have published a paper on the new hydrogen storage system in a recent issue of the Journal of the American Chemical Society.
As the researchers explain, hydrogen has a very high mass energy density but a very low volumetric energy density. The high mass energy density, which is at least three times higher than that of other chemical fuels, is what makes hydrogen such an attractive energy carrier. However, its low volumetric energy density under ambient conditions makes it difficult to store large amounts of hydrogen in small spaces. To overcome this problem, hydrogen is often stored at high pressures or low temperatures, but these storage methods present their own challenges.
The hydrogen storage system demonstrated in the new paper works under ambient conditions and stores the hydrogen in abundant, lightweight, and inexpensive molecules called hydrocarbons. The researchers demonstrated that six hydrogen atoms can be added to benzene (C6H6) in a "hydrogenation" process that forms cyclohexane (C6H12), which serves as the hydrogen carrier. In the reverse process, cyclohexane is "dehydrogenated" as the six carbons are removed and available for use in energy storage devices and other applications.
This method of storing hydrogen atoms in hydrocarbons is not new, but because the dehydrogenation process requires a large amount of energy to proceed, current versions always require high temperatures to release the hydrogen.
Since performing the reaction at high temperatures is not suitable for practical applications, here the researchers demonstrated that solar energy can be used to drive the dehydrogenation reaction at ambient temperatures. This process involves using platinum-based nanoparticles as photocatalysts. After absorbing incoming photons, the platinum nanoparticles temporarily donate their photoexcited electrons to the cyclohexane molecules, breaking the carbon-hydrogen bonds and releasing the hydrogen atoms without the need for elevated temperatures.
Tests showed that this photo-driven dehydrogenation process occurs rapidly (within a few seconds), converts 99% of the cyclohexane to benzene, and has a quantum efficiency (H2 produced per photon consumed) of 6.0%, which rivals the current top-performing solar water splitting devices without an external voltage. To start the hydrogenation process, the researchers simply removed the light source, causing the hydrogen atoms to reattach back onto the benzene. Using this method, 97% of the benzene could be converted back to cyclohexane, and the cycle could be repeated.
The researchers expect that this strategy is more suitable for stationary applications—for instance, for storing and transporting energy produced by wind turbines or other alternative energy sources—rather than vehicles because of the fact that it requires sunlight to release the hydrogen.
"The applications may include the storage and transport of hydrogen generated from other sources, such as water splitting and water electrolysis, using renewable energies (hydro, wind, nuclear, etc.)," Li told Phys.org.
Taking the next steps forward, McGill University has filed a provisional patent on this technology. In the future, the scientists plan to improve the hydrogen storage system by reducing the amount of platinum required in the photocatalysts and developing other less expensive alternatives.
"Our future research is focused on developing cheaper and more earth-abundant metal catalysts, such as iron, and to further increase the quantum efficiency," Li said.
More information: Lu Li, et al. "Simple and Efficient System for Combined Solar Energy Harvesting and Reversible Hydrogen Storage." Journal of the American Chemical Society. DOI: 10.1021/jacs.5b03505
Journal information: Journal of the American Chemical Society
© 2015 Phys.org




















