September 3, 2015 report
Macrophages create the elusive spermatogonial stem cell niche
(Phys.org)—Every organ strikes its own balance between self-renewal and differentiation. At one extreme is the brain, where only a few isolated outposts are known to contribute to a largely quiescent population. At another extreme are the testes, where at least in males, prolific germline turnover is maintained amidst a protracted and deliberate multi-month creation cycle. Perhaps surprisingly, both organs are uniquely immunoprivileged against various vascular indiscretions and yet rely on various myeloid-derived cells like macrophages or microglia to instruct important structural maturations.
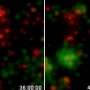
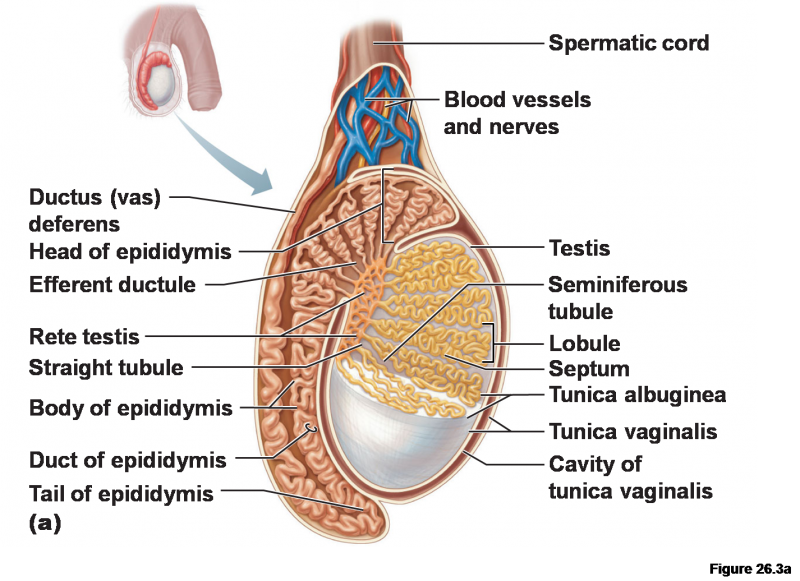
The so-called stem cell 'niche' is the microenvironment of the stem cell: all those physical and molecular intangibles that nourish, shield and inform its major life decisions. In creatures like worms and flies a well-defined stem cell niche is distally localized within a polarized gonad. By contrast, each testes of the mammal can contain up to 40,000 potential stem cell niches more-or-less randomly dispersed among its seminiferous tubules. A recent paper published in Cell Reports has removed some of the mystery in the mammalian reproductive system by showing that a unique class of testicular macrophages define its putative stem cell niche.
Perhaps even more importantly, they also show that these macrophages control spermatogonial differentiation through the expression of critical factors including CSF1 (colony-stimulating factor) and retinoic acid synthesis enzymes. When the researchers ablated the macrophages by making them selectively and transiently susceptible to the diphtheria toxin, they found a drastic reduction in the number of spermatogonia. This new found responsibility of macrophages complements their previous known testicular functions in promoting steroidogenesis in Leydig cells. They are also known to be intimately involved in vascular development in several places, including the brain. We should mention that the immune barrier in the brain is due mainly to the endfeet of astroglial cells while in the testes it is created by tight junctions in the Sertoli cells that nurse the developing spermatogonia.
The single spermatogonium is the most undifferentiated cell within the prevailing model of the spermatogonial stem cell hierarchy. In the fullness of time each one progresses through an incomplete cytokinesis, giving rise to syncytial cysts of spermatogonia progeny that are held together in formation by cytoplasmic bridges. In this way those cells in the syncytium that lack and X chromosome can still get copies for things like glucose 6 phosphate dehydrogenase. Similarly those cells that find themselves without a Y still have access to essentials like the RBM gene, which is critical later in spermatogenesis.
For most men, the only time they ever hear the word macrophage in reference to their testicles is when it comes time for the vasectomy. Upon being told during that actual sperm production is totally unaffected by a vasectomy, any reasonable man might be expected to wonder, perhaps aloud, 'what happens to all the sperm'? Typically there is some mumbling about macrophages taking care of all that, although the actual details are a bit shoddy. This new evidence regarding the intimate relationship between macrophages, spermatogenesis, and perhaps even spermicide, points to the indebtedness we have to these dynamic cells.
Since we have already broached the subject we might take the opportunity to share a few more illuminative details that may benefit anyone undergoing significant alterations to their reproductive plumbing. While a general attitude of 'the less you know, the better' may often prevail in such matters, there are some things a patient should probably know. In up to half of patients something known in the business simply as 'epididymal blowout' will occur. This is something every bit as horrid to imagine as it sounds. Typically, pressure in the vas deferens (which usually is tied off or electrocauterized) builds up over the course of several weeks post-op to the point that it ruptures, frequently accompanied by some pain and possible formation of epidydimal cysts. Fortunately, through a cooperative macrophage effort, this situation largely resolves on its own.
However for some men something even more sinister lurks within. Another word that can bring even the strongest to their knees is 're-canalization'. This situation is also precisely as it sounds—complete regrowth and functional connection of a severed vas deferens. It is not yet known exactly how or when this can occur other than to say that 'where there's a will there's a way'. For men lucky enough to avoid any of these potential pitfalls there is probably only one remaining concern: what happens to levels of testosterone?
Studies have apparently shown that testosterone levels remain the same on average. What such a devilish statement hides is that depending on your particular constitution and sequalae, you might your own individual expect levels to change, either for the better or for worse. In other words, with evidence in literature for both increases and decreases, it is fair to say we need more research on how such things are ultimately controlled in the gonads.
More information: The New Director of "the Spermatogonial Niche": Introducing the Peritubular Macrophage, Cell Reports, Volume 12, Issue 7, p1069–1070, 18 August 2015. dx.doi.org/10.1016/j.celrep.2015.07.057
Macrophages Contribute to the Spermatogonial Niche in the Adult Testis, Cell Reports, Volume 12, Issue 7, p1107–1119, 18 August 2015. dx.doi.org/10.1016/j.celrep.2015.07.015
Journal information: Cell Reports
© 2015 Phys.org