February 17, 2016 report
New hydrogel nanoplatform that identifies and shrinks tumors in mice
(Phys.org)—Functionalized nanoparticles are one avenue of drug delivery for chemotherapeutics. However, getting nanoparticles to target the tumor site has proven difficult to do. One method researchers have used to target cancer cells is to create hydrogels made of filamentous bacteriophage (phage) and gold nanoparticles. Peptide binding ligands can be incorporated into the phage portion of the hydrogel that will then target known carcinoma cells.
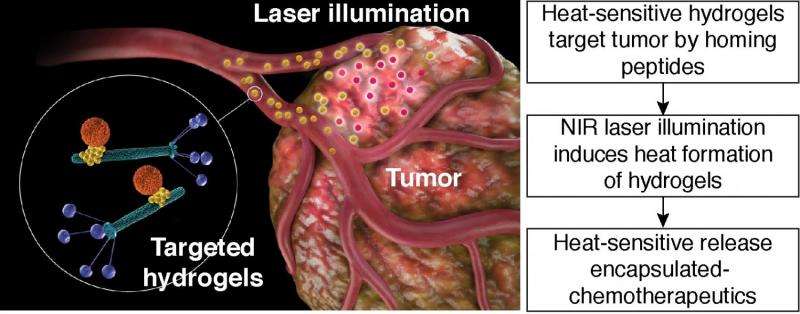
An interdisciplinary group of researchers from various universities in Japan, the U.S., and Germany have developed a hydrogel platform that can target tumors, provide non-invasive imaging, and release a chemotherapeutic drug. They have demonstrated their platform in both in vitro studies and in mouse models. Additionally, they demonstrate that their platform is generalizable to different targets and chemotherapeutics. Their work appears in the Proceedings of the National Academy of Sciences.
Hosoya, et al.'s hydrogel platform consists of bacteriophage, gold nanoparticles, and nano-sized carriers such as liposomes or mesopourous silica particles. In this setting, phage particles are able to recognize specific molecules on the tumor cells. The gold nanoparticles serve as a "reporter" for the distribution of the hydrogel. The nanocarrier transports various chemicals or pharmaceutical cargo. The nanocarrier releases cargo upon a specific stimulus, such as heat.
In the current work, Hosoya, et al. first proved that heat-sensitive liposomes (HSLs) would serve as a formidable heat-sensitive carrier. In theory, as the HSL begins to melt, it releases the drug that is incorporated into it. Using calcein, a fluorescently active molecule, they determined that HSL released calcein upon reaching 40oC, as predicted. When the HSL was left at a constant temperature (42oC), it released all of the calcein within 10 minutes. Hosoya, et al. then proved that these HSLs were still responsive to changes in temperature when incorporated into the hydrogel platform.
The next step was to demonstrate that the HSL-containing hydrogels responded to NIR heating while within a matrix. The authors used agarose gel as their model system. They found that the HSL-containing hydrogel responded to NIR heating and as laser power increased, the temperature of the hydrogel increased. They then determined whether NIR heating would trigger the release of doxorubicin (dox), a chemotherapeutic. They were able to produce a "reproducible and robust photon dose-dependent increase in fluorescence intensity."
To determine the heat distribution in the hydrogel from NIR, they used magnetic resonance temperature imaging on the HSL-containing hydrogels on the agarose platform. The thermal gradient images confirmed that the centralized heat was produced by the hydrogel via the NIR laser. They used gadolinium-encapsulated HSL-containing hydrogels to confirm that drug release occurred at the location of laser beam.
While these results show that drug release can be controlled using NIR, they still need to test whether the system can target the cancer site. To do this, Hosoya, et al incorporated a ligand that has a well-established cyclic peptide that binds to CRKL. CRKL-binding phage particles target EF43.fgf-4 mammary carcinoma cells. Using rhodamine-labeled HSL-containing hydrogels, they demonstrated that the phage targeted the carcinoma cells, confirming that it still maintains its binding properties even when incorporated into the nanoplatform.
They then did in vivo testing in mice. They tracked the location and effects of their HSL-containing hydrogel platform in mice that had EF43.fgf-4 mammary carcinoma. Optical fluorescence imaging studies showed the tumor was visible in mice treated with the targeted hydrogel system compared to controls. Analysis of the tumors after 24 h revealed that gold nanoparticles, targeted phage, and HSLs were located within the tumor. Additional studies to see if their system could then release a chemotherapeutic using NIR at the tumor site also proved successful. The authors observed reduced tumor growth in mice with HSL-containing hydrogels with dox, and confirmed their results using mathematical modeling.
Finally, to test the versatility of their hydrogel nanoplatform, Hosoya, et al. incorporated mesoporous silica nanoparticles (MSNP) into the hydrogel system. MSNP's chemical properties allow for packing different chemotherapeutics. They tested whether MSNP-containing hydrogels could deliver FITC in two different cancer models, the same breast cancer model used previously (EF43.fgf-4) and a model for prostate cancer. They found targeted binding to the two cancers and studies with dox showed a decrease in tumor size.
This work demonstrates a hydrogel platform that does not change the physical or chemical properties of known nanocarrier systems such as heat-sensitive liposomes or mesoporous silica nanoparticles. This platform allows for targeting, heat-induced delivery, and is both versatile and reproducible. With additional studies, this system could be a general, robust method for targeted drug delivery.
More information: Hitomi Hosoya et al. Integrated nanotechnology platform for tumor-targeted multimodal imaging and therapeutic cargo release, Proceedings of the National Academy of Sciences (2016). DOI: 10.1073/pnas.1525796113
Abstract
A major challenge of targeted molecular imaging and drug delivery in cancer is establishing a functional combination of ligand-directed cargo with a triggered release system. Here we develop a hydrogel-based nanotechnology platform that integrates tumor targeting, photon-to-heat conversion, and triggered drug delivery within a single nanostructure to enable multimodal imaging and controlled release of therapeutic cargo. In proof-of-concept experiments, we show a broad range of ligand peptide-based applications with phage particles, heat-sensitive liposomes, or mesoporous silica nanoparticles that self-assemble into a hydrogel for tumor-targeted drug delivery. Because nanoparticles pack densely within the nanocarrier, their surface plasmon resonance shifts to near-infrared, thereby enabling a laser-mediated photothermal mechanism of cargo release. We demonstrate both noninvasive imaging and targeted drug delivery in preclinical mouse models of breast and prostate cancer. Finally, we applied mathematical modeling to predict and confirm tumor targeting and drug delivery. These results are meaningful steps toward the design and initial translation of an enabling nanotechnology platform with potential for broad clinical applications.
Journal information: Proceedings of the National Academy of Sciences
© 2016 Phys.org