October 9, 2015 report
Water-soluble non-peptide foldamers with tunable higher-order conformations
"Foldamers" are a class of synthetic structures modeled after proteins, but are designed to form unique, non-natural tertiary and quaternary structures. Like proteins, they are comprised of hydrophobic and hydrophilic subunits, but unlike proteins, they are not made with the twenty naturally-occurring amino acids. Foldamers are attractive for bioapplications, but controlling their higher-order folded structure has proved difficult. Furthermore, while studies on foldamers have been done in non-polar and moderately polar solvents, researchers have made little headway in designing aqueous foldamers.
A collaboration of researchers from the Universite de Bordeaux, CNRS, and INSERM in France have designed unique oligourea helical foldamers that self-assemble in aqueous conditions and whose quaternary structures can be controlled by changing the hydrophilic and hydrophobic profiles of their primary sequences. These foldamers can be tuned to yield a distinct hydrophobic cavity or a pH-tunable channel with water-filled pores. Their structural features have intriguing implications for pharmaceuticals as well as cross-membrane ion conductance. This research appears in Nature Chemistry.
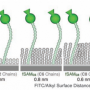
Collie, et al.'s new foldamers were designed with an oligourea backbone (i.e., a sequence comprised of a urea backbone and amino acid side chains). Prior studies with oligoureas show that they can form helical structures that are relatively unaffected by the nature of the side chains. The two foldamer models in this study only differed in their hydrophobic and hydrophilic profiles. The aim was to use these varying profiles to guide self-assembly into quaternary structures with regions of hydrophobicity and hydrophilicity in aqueous solution. After synthesis of their two oligourea sequences, circular dichroism (CD) studies indicated concentration-dependent helix formation in water, a promising sign that they will form a higher-order structure.
Crystal studies of one of the oligoureas (H1) indicated six helices bundled together. This bundle has a hydrophobic core with leucine side chains oriented toward the core and interlocked similarly to the "knobs-into-holes" packing structure seen in α-helix bundles. This hexameric structure has not been reported for aqueous foldamers before. Two unexpected features of this unique structure were its lack of salt bridges even though each helix had charged residues that would likely form a salt bridge, and the presence of an isolated cavity within the hydrophobic core. The cavity's size (495.0 Å3) makes it an intriguing candidate for harboring a guest molecule.
Characterization of the second oligourea structure (H2) indicated a highly-charged, water-filled channel assembly with a hydrophobic exterior. Additionally, H2 has interhelical salt bridges, which the authors believe play an important role in stabilizing the channel structure. Indeed, additional studies showed that at a pH low enough to prohibit the formation of salt bridges, H2 forms a monomeric helix rather than a channel.
Additional oligoureas were made with slight changes to see how they compare to their H1 or H2 counterparts. H3 is an oligourea that is analogous to H1, but one of the key hydrophobic residues was replaced by a hydrophilic residue (asparagine). If self-assembly is truly guided by hydrophobic and hydrophilic interactions, then this replacement should inhibit self-assembly. ESI-MS and high-field NMR studies confirmed that H1 was a hexamer while H3 was monomeric, lacking the specific intermolecular interactions one would expect with a hexameric structure.
Additionally, Collie, et al. made oligourea H4 in which the position one residue, leucine, in H1 is replaced with serine. This residue was not part of the knob-into-hole assembly, so the oligourea should self-assemble to a similar higher-order conformation as H1. Characterization showed that H4 formed a hexameric helical bundle similar to H1, but with some additional interhelical interactions. Importantly, H4 formed a hydrophobic cavity that was slightly smaller than the H1 cavity, demonstrating that the cavity size may be tunable.
Finally, to see how the oligourea quaternary conformations can be tuned, a fifth oligourea (H5) was made with uncharged residues at three continuous helical 'pentad positons' and a continuous charged helical face. Because the quaternary structure seems to rely on how charged and uncharged residues are distributed in the oligourea, H5 was designed to see if one could control whether the oligourea self-assembles into a helical bundle or tubular nanostructure. Collie, et al. found H5 had a channel-structure similar to H2 and studies showed a pore diameter that is slightly larger than the H2 channel structure, indicating that the channel size may be tunable.
This research shows a straight-forward process to tailor non-peptide foldamers into either helical bundles or water-filled channels with tunable diameters. The bundles have a hydrophobic cavity that could be used for drug or cargo entrapment or host-guest chemistry while the channels could be used for ion transport. These structures overcome many of the limitations of foldamer chemistry, and offer a potentially generalizable method for making practical structures.
More information: Gavin W. Collie et al, Shaping quaternary assemblies of water-soluble non-peptide helical foldamers by sequence manipulation, Nature Chemistry (2015). DOI: 10.1038/NCHEM.2353
Journal information: Nature Chemistry
© 2015 Phys.org