March 14, 2016 report
Water dimer captured inside a fullerene-C70
(Phys.org)—Researchers from Kyoto University have, for the first time, isolated a water dimer. Using a technique known as molecular surgery, they encapsulated the dimer within a fullerene-C70 molecule. Their work appears in the recent issue of Nature Chemistry.
Water has some rather unique properties. For example, water has an exceptionally high melting and boiling point thanks to its intermolecular hydrogen bonding network. It is this bonding network that makes isolating two water molecules very difficult. Investigating a water dimer would allow researchers to gain further insight into water's intermolecular bonding network. Furthermore, water dimers have been observed in atmospheric water vapor.
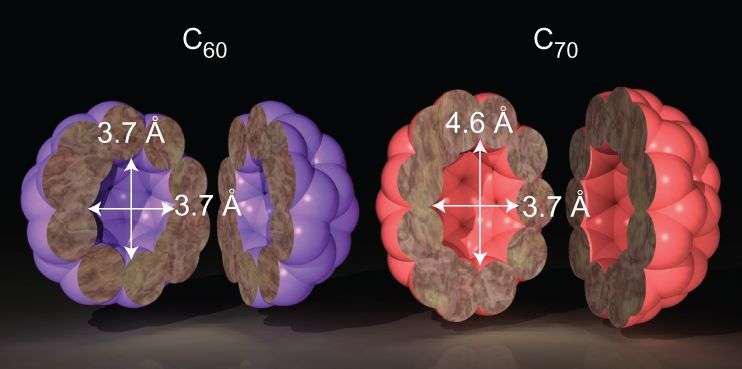
Prior studies have successfully isolated a single water molecule within a fullerene-C60. Fullerenes are hollow carbon spheres whose interior is isolated from the surrounding environment. Researchers have isolated highly reactive species within a fullerene such as metals or a nitrogen atom. The interior cavity of the fullerene-C60 is 3.7Å in diameter, which is too small to house a water dimer so Rui Zhang, Michihisa Murata, Tomoko Aharen, Atsushi Wakamiya, Takafumi Shimoaka, Takeshi Hasegawa, and Yasujiro Murata from Kyoto University focused on fullerene-C70, which has an ellipsoid shape with a long diameter of 4.6 Å.
Zhang, et al. used a technique, known as molecular surgery, to insert one and two water molecules into a fullerene-C70. The molecular surgical method involves making a "hole" in a fullerene-C70 via a chemical reaction, inserting the target molecules under extreme conditions, and then closing the hole to reform the C70 sphere. Zhang, et al. developed a novel way to open the fullerene-C70 cage.
There are two reactive sites on the surface of a fullerene-C70, α and β bonds that are good candidates for alkene addition. While α bonds are more reactive, focusing the addition reaction on the β bonds yielded a larger pore size. Once Zhang, et al. achieved a sufficiently large pore size through sequential C=C cleavage, they then forced water into the fullerene cavity under high pressure (9,000 atm) and heat (120oC).
They were able to restore the fullerene's cage using a two-step process and isolated fullerene-C70 with a single water molecule from empty fullerene using HPLC. Proton NMR and atmospheric pressure chemical ionization mass spectrometry confirmed the presence of a single water molecule within the fullerene. X-ray diffraction studies indicated that the water molecule is off-center within the cage and that there is still pore space available for the possibility of an additional water molecule.
Indeed, Zhang, et al. found a trace product that they isolated using HPLC. Mass spectrometry and NMR studies provided compelling evidence that this was a water dimer encapsulated by fullerene-C70. The dimer has a single hydrogen bond between and the two molecules adopted a different conformation (cis-linear) from water's normal conformation (trans-linear). Additionally, infrared studies clearly distinguished the empty C70 from the C70 containing one water molecule and the C70 containing two water molecules, providing further insight into the differences between the three species.
This research provides an excellent opportunity for further studies to understand this water's unique conformation in a confined space, as well as the nature of the single hydrogen bond.
More information: Rui Zhang et al. Synthesis of a distinct water dimer inside fullerene C70, Nature Chemistry (2016). DOI: 10.1038/nchem.2464
Abstract
The water dimer is an ideal chemical species with which to study hydrogen bonds. Owing to the equilibrium between the monomer and oligomer structure, however, selective generation and separation of a genuine water dimer has not yet been achieved. Here, we report a synthetic strategy that leads to the successful encapsulation of one or two water molecules inside fullerene C70. These endohedral C70 compounds offer the opportunity to study the intrinsic properties of a single water molecule without any hydrogen bonding, as well as an isolated water dimer with a single hydrogen bond between the two molecules. The unambiguously determined off-centre position of water in (H2O)2@C70 by X-ray diffraction provides insights into the formation of (H2O)2@C70. Subsequently, the 1H NMR spectroscopic measurements for (H2O)2@C70 confirmed the formation of a single hydrogen bond rapidly interchanging between the encapsulated water dimer. Our theoretical calculations revealed a peculiar cis-linear conformation of the dimer resulting from confinement effects inside C70.
Journal information: Nature Chemistry
© 2016 Phys.org