Development and clinical approval of biodegradeble magnesium alloy
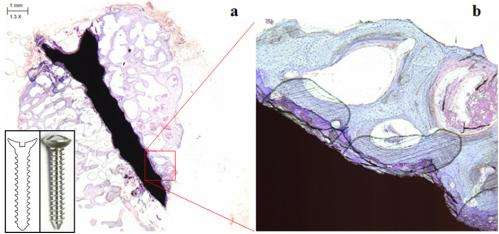
This biodegradable and bioabsorbable metal decomposes from 6 months to 2 years after being transplanted into human body and hence, medical devices made with these materials are expected to reshape the landscape in the field of fracture treatment, as it reminders second operation to take out the device after patient recovery obsolete. KIST Consortium Consortium participated by U & I (Co.), ASAN Medical Center and Seoul National University (led by Dr. Seok, Hyun-kwang of KIST Biomedical Research Institute) succeeded in developing high strength/low biodegradable metal made from basic elements and minerals essential to human body.
The Consortium also developed biodegradable and bioabsorbable medical devices for use in orthopedics & plastic surgery while earning approval to clinically test the device. The clinical trial is currently in progress conducted by Ajou University Medical Center. The heart of the research is matching potentials between the matrix structure of metal and the secondary agents on the matrix structure to overcome the fundamental limitation of metal materials, which is speedy degradation. Using this application, new innovative materials can be developed such as metal alloy with the 2nd and 3rd additional elements while still maintaining the electrochemical nature of pure metal. The remarkable achievement was made possible thanks to the Computational Materials Science Laboratory at Kookmin University (led by Professor Cha Phil-ryung), which helped to create the effects of synergy between computer simulation and technology. With extensive support of the Seoul Strategic Industry Initiative, the key support program of the Seoul City government for Smaller enterprises' R&D, KIST Consortium has succeeded in developing new degradable metal materials inside human body after transplant, and also received approval to clinically test these metals from the Korea Ministry of Food and Drug Safety as a bioabsorbable fixation device to be used in orthopedics and plastic surgery. KIST was selected as a beneficiary for the Seoul Strategic Industry Support Initiative in 2010 and has been working on the project for 3 years. Further, these findings were published in the 2013 August edition of Scientific Report, a sister magazine of the world-renowned scientific journal, Nature.
Provided by Korea Institute of Science and Technology