Clinical trial: Patient receives a 3D-bioprinted ear implant grown from their own cells
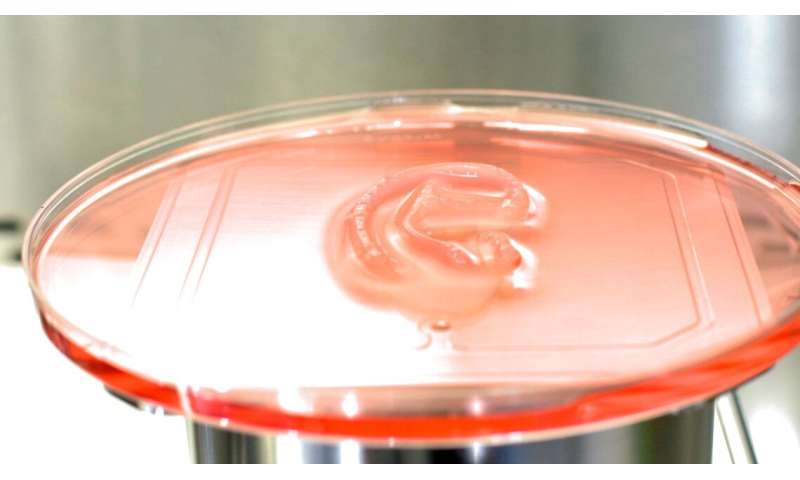
In a first-of-its-kind clinical trial, a human has received a 3D-bioprinted ear implant grown from the patient's own living cells—thanks to a technology platform developed by a Cornellian-founded startup company.
This bioengineered breakthrough has the potential to significantly improve the lives of the approximately 1,500 children who are born annually in the U.S. with microtia, a congenital ear deformity. The approach could eventually lead to tissue implants for treating other conditions and traumatic injuries, reconstructive and regenerative therapy, and possibly even the biomanufacture of whole organs.
The company, 3DBio Therapeutics, was founded in 2014 by Dan Cohen, along with Lawrence Bonassar, the Daljit S. and Elaine Sarkaria Professor in Biomedical Engineering and in Mechanical and Aerospace Engineering in the College of Engineering, and Hod Lipson, who taught at Cornell for 14 years and is now a professor at Columbia University.
The platform developed by 3DBio Therapeutics consists of an entire suite of technologies, processes and engineering that supports the 3D bioprinting.
"Not only is this an application you've never seen before, it's made with a technology you've never seen before," Bonassar said. "Even tissue-engineered implants in general, there just aren't that many of them on the market, or even in clinical trials."
The process begins with a biopsy from which chondrocytes—cells that form cartilage—are extracted from a patient's impacted ear. The cells are expanded in a specialized cell culture system and then mixed with a collagen-based bio-ink, called ColVivo. That material is shaped via a 3D bioprinter into a living ear implant that matches the size and shape of a typical ear. This AuriNovo implant is surgically placed under the patient's skin along with a temporary biodegradable shell that provides protection and structural support.
'An interdisciplinary, collaborative environment'
The project began in the early 2000s, when Cohen was an undergraduate working in Lipson's lab on 3D printing nontraditional materials. Bonassar came to Cornell in 2003 after five years on the faculty of the Center for Tissue Engineering at the University of Massachusetts Medical School, where he had worked with pioneering tissue engineer Charles Vacanti, who was part of the team that made international headlines in 1997 when they implanted cartilage shaped like a human ear onto the back of a mouse. At the recommendation of his department chair, Bonassar met with Lipson and Cohen, who shared his interest in exploring 3D printing.
"In that meeting, we realized that we could potentially print the cell-seeded hydrogels that I was working with in my lab, using some of the printing technology that was being developed in Hod's lab," Bonassar said. "It's that interdisciplinary, collaborative environment that is a signature and a brand of Cornell. It required this out-of-the-box thinking. Hod is a roboticist who was making autonomous robots, and I'm a bioengineer. Put us together and we invent one thrust of bioprinting, and it got put into action by an ambitious, talented undergrad who was looking for a cool project."
In February 2013, a collaboration between Bonassar's lab and Weill Cornell Medical College physicians published an early version of a 3D-bioprinted ear using collagen derived from cartilage cells of animals.

Ever since that research was published, Bonassar has received hundreds of emails from parents asking if the technology was available yet.
"I've been getting emails from patients for a decade. And it's really heartbreaking," he said. "They are talking about their little baby and saying "Can we get this?" And to be honest, the surgeons would like other options."
There are two current treatments for microtia. The first involves surgically harvesting rib cartilage from a patient, carving the cartilage into the shape of an ear and then creating a pocket of skin on the side of the patient's head that will hold the newly shaped ear. The second approach uses plastic implants, usually made from porous polyethylene, that are implanted in a similar fashion. Now, the 3D-bioprinting platform potentially offers patients an alternative solution with a living ear implant made from their own cells.
'A triumph of engineering'
Cohen had continued to work on the project throughout his master's and doctoral degrees. After earning his Ph.D. in 2010, he joined the management consulting firm McKinsey & Company.
"I focused on lots of different types of projects in many different industries. I joined the corporate finance practice at McKinsey, and had an opportunity to experience firsthand the broader business context surrounding technology companies," he said. "But after four years, I realized there was an opportunity to try to bring the technology to clinic. I approached Larry and we sat at his kitchen table along with Hod, and the three of us discussed how we might go ahead and proceed as a company."
The leap from university research to a commercial technology represents almost eight years of 3DBio's efforts and has required a whole string of new innovations, from addressing cell expansion to developing therapeutic-grade bio-ink, all with an eye toward meeting the Food and Drug Administration's requirements—known as Current Good Manufacturing Practice—for producing a safe medical product.
The phase 1/2a clinical trial, which involves 11 patients and is currently underway, aims to evaluate the safety and preliminary efficacy of the technology specifically for patients with microtia. The ear reconstruction was performed by the Microtia-Congenital Ear Deformity Institute in San Antonio, Texas, by Dr. Arturo Bonilla. The second clinical trial site is Cedars Sinai in Los Angeles, with Dr. John Reinisch.
The fact that the project has reached this milestone, Bonassar said, is the result of the researchers' clear vision from the very beginning: to make a clinically functional implantable tissue, one that is focused purely on cartilage's mechanical task.
"This is really a triumph of engineering," Bonassar said.
For Bonassar and Cohen, the clinical trial has been a long time coming.
"3DBio has successfully taken over where academia leaves off and has truly industrialized the technology," Cohen said. "Oftentimes with novel technologies it takes a while for fundamental technical hurdles to be leaped. Then it's followed by fast-paced progress. We've seen this in aviation, computing and many other fields. Now that we believe we've cleared those fundamental hurdles, this technology platform could potentially make an impact in the lives of patients, and be the start of a new treatment paradigm."
Provided by Cornell University