Role of mutation in nucleoprotein SARS-CoV-2
Scientists from The Federal Research Centre "Fundamentals of Biotechnology" of the Russian Academy of Sciences together with foreign colleagues demonstrated that human 14-3-3 proteins, that are known for their role in replication of many viruses, bind differentially with more often mutating regulatory part of nucleoprotein (N protein) of coronavirus SARS-CoV-2. Presumably, the result of this correlation changes both the virus life cycle and 14-3-3-dependent cell functions. The interaction force of the 14-3-3 and N protein is greatly influenced by mutations in the particular parts of the latter; therefore, the results of the research, published in the Journal of Molecular Biology, may be useful in drug discovery against new strains of coronavirus. Research is supported by the national project "Science and Universities".
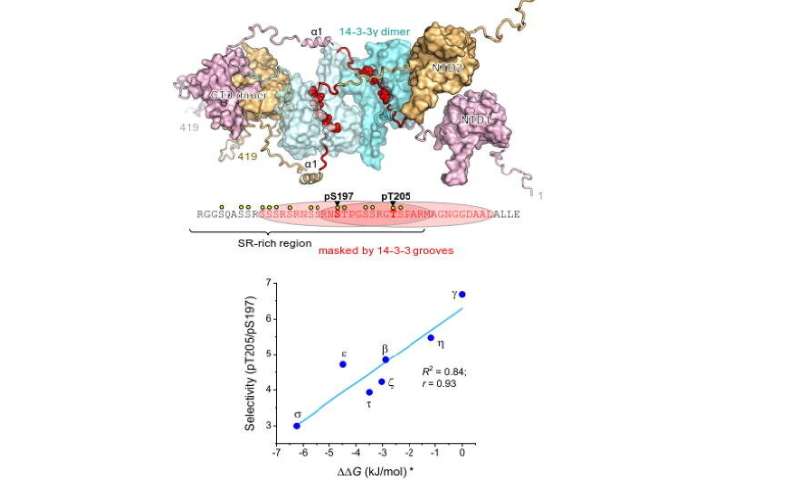
Nucleocapsid protein (nucleoprotein or N protein) is common for single-stranded RNA-viruses, including coronaviruses, and is responsible for replication, packaging and storage of viral genome. Its structure has a central regulatory part, consisting of about 30 amino acid residues (mainly the residues of serine and arginine, the so-called SR-rich region), where special cellular enzymes transfer phosphate groups from molecules of ATP (phosphorylate them). Such modifications trigger human 14-3-3 proteins to bind N-protein. 14-3-3 proteins participate in a range of crucial cell processes: regulate the activity of the protein partners, their intracellular distribution, and their interaction with each other, thus becoming involved in the regulation of cell cycle, metabolism, gene activity, and cell death (apoptosis).
"In our previous work, we demonstrated that 14-3-3 proteins recognise the nucleocapsid protein of SARS-CoV-2, and we were able to determine the precise area of their interaction. Now we decided to check whether other similar areas in N protein exist", explains Kristina Tugaeva, the first author of the work, the member of the group "Protein-protein interaction" of the Federal Research Centre "Fundamentals of Biotechnology" of the Russian Academy of Sciences.
This task is important since the 14-3-3-binding part of N protein is located in the SR-rich region, which is a hotspot of viral mutations. If in the case of S protein the consequences of mutations seem obvious—they make virus entry into the cell easier or help evade the immune system, whereas the effects of mutations in N protein remain mainly unknown, in spite of the fact that N protein is the main factor of pathogenicity.
The authors found that 14-3-3 proteins site-selectively recognize to either of two phosphorylated pseudo-repeats in the SR-region of the SARS-CoV-2 nucleoprotein: centred at Ser197, identified earlier, and a new site, centred at Thr205. Interestingly enough, the binding force (affinity) of the second area turned out to be tighter for all members of the 14-3-3 family.
Structural insights led to the conclusion that the Ser197 and Thr205 residues in the N protein are located too close to each other to allow 14-3-3 to bind both. Thanks to the interaction with 14-3-3, the regulatory SR-region of the N protein could be protected from cell enzymes that can influence the cell life cycle by removing phosphate groups.
"So we suggested that mutations in the N protein of the coronavirus affect the binding efficiency of 14-3-3. Moreover, precisely those disordered regions especially sensitive to mutations play a role in this interaction. The results of our new research could contribute to the discovery of drugs against new strains of coronavirus", concludes Kristina Tugaeva.
More information:
Kristina V. Tugaeva et al, Human 14-3-3 Proteins Site-selectively Bind the Mutational Hotspot Region of SARS-CoV-2 Nucleoprotein Modulating its Phosphoregulation, Journal of Molecular Biology (2022). DOI: 10.1016/j.jmb.2022.167891
Provided by Immanuel Kant Baltic Federal University