Experimental model gets cells to behave as they would in utero
Many birth defects and spontaneous abortions occur during the embryonic development stage known as neurulation, yet we have very little insight into how this critical developmental process unfolds in humans.
The Rice University lab of Aryeh Warmflash has received a five-year, $1.9 million grant from the National Institutes of Health to optimize and develop experimental cell models that can shed light on the self-organizing processes by which ectodermal cells give rise to the central nervous system, skin and sensory organs.
The Warmflash lab will use a technique called micropatterning to get colonies of ectodermal cells to self-organize and differentiate in ways that mirror embryonic development. By engineering the cells to express different fluorescent cues based on the type of protein signal they use to communicate, scientists will be able to track and quantify the cells' self-organizing behaviors.
The research could inform ways to prevent or counteract central nervous system birth defects stemming from anomalous neurulation.
"The most basic question we're interested in is what guides the cells' behavior to assemble into the tissues of the embryo," said Warmflash, an associate professor of biosciences and Cancer Prevention and Research Institute of Texas scholar.
To study this question, Warmflash and collaborators used micropatterning and cultured human pluripotent stem cells to develop self-organizing systems that model key cellular processes involved in embryogenesis.
"Micropatterning technology is just a way of controlling very precisely the geometry in which these colonies of cells grow," Warmflash said. "The micropatterning allows us to prepattern the surface with different chemical modifications so that we can say, 'OK, we want the cells to stay here and not there.'"
In a standard stem cell culture, cells will grow into clumps of different sizes and differentiate randomly into a disorganized, extremely heterogeneous mass. But if micropatterning is used to curb random growth, the cells behave in an organized fashion that mirrors the behavior of cells in an actual embryo.
"Mammalian development is a self-organizing process," Warmflash said. "What this means is that, initially, you grow this ball of cells that's a perfectly symmetrical structure because all the cells in it are basically the same.
"Then there are organisms where that symmetry is already broken. For example, when the mom fly makes a fly egg, that egg is already asymmetrical: Just by looking at it, you can know where the head is going to be, etc.
"In the mammalian or human embryo, that symmetry is initially unbroken. But eventually, the embryo has to figure out 'this side is going to be the head and this is going to be the back' and so on. The cells have to break that symmetry themselves. We want to understand how they do that."
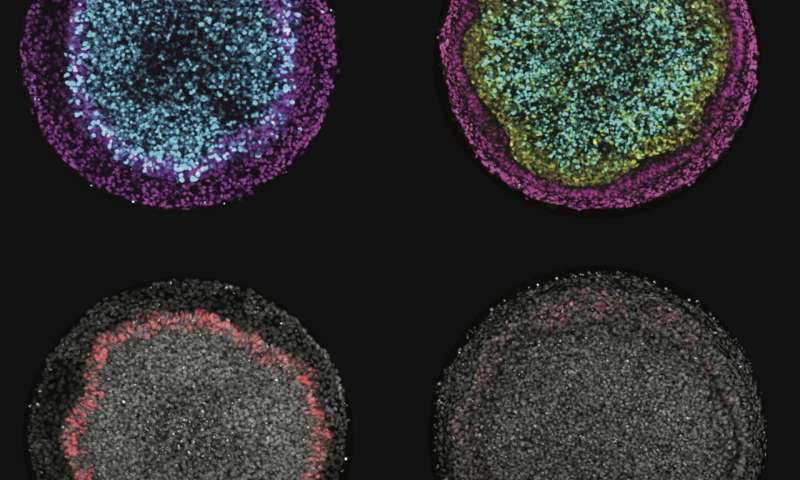
In an embryo, ectodermal cells sort themselves into one of four cellular "fates" as either nervous system, neural crest, skin or sensory organs. With micropatterning, Warmflash and his team were able to manipulate intracellular signals so as to attain a distribution of cellular fates in a bull's-eye pattern that mimics ectodermal patterning along the medial-lateral axis of the embryo.
"We induce the cells to differentiate with patterning signals, and then they figure out how to arrange themselves," Warmflash said. "They say, 'OK, we have to make these four different fates—the neurons are going to be at the center and the skin is going to be at the edge, with the other fates in between.' That's the midline-to-side axis, but there's another axis forming roughly simultaneously, which is the head-to-toe axis.
"Right now, there really aren't good models for how the head-to-toe axis of the nervous system forms in humans, which patterns the nervous system into very broad regions: a forebrain, the midbrain, hindbrain and then the spinal cord."
Warmflash hopes to fine-tune the system in order to model the head-to-toe developmental axis, "where instead of neurons versus skin at the edge of the colony, the center of the colony will be forebrain neurons and to the edge of the colony there will be spinal cord neurons.
"Instead of representing different types of ectoderm arrayed along the medial-lateral axis, the colony will represent the nervous system arrayed along the longitudinal axis of the embryonic structure. We're interested in how cells communicate to do that."
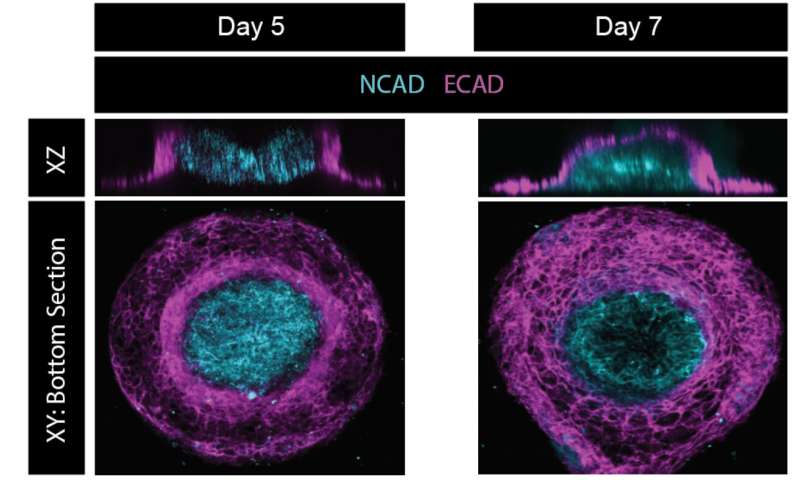
In addition to these 2D maps of cellular patterns of self-organization, Warmflash hopes to use the micropatterning modeling system to study morphogenesis, which refers to how a tissue or organ occupies space and develops its specific shape.
"In this ectoderm model, you can induce the cells to fold in the third dimension," Warmflash said. "And they'll actually close over the top in a way that's very similar to the way the neural tube closes during development. We're interested in this because you have signaling between cells which both changes the type of cell they become and also influences how they move and are sculpted to actually make a functioning tissue.
"It's possibly very clinically relevant because many birth defects are actually neural tube closure defects."
The modeling system could be used to study mutations that cause neural tube closure defects, opening up the possibility of figuring out how to prevent or remedy such events.
"It's not really known how particular mutations lead to particular defects," Warmflash said. "But those are things that we can study in these models with the goal of, first, understanding how things break and, then, hopefully figuring out how to prevent those things from happening."
Provided by Rice University