NLRP3 acts as a direct driver of hepatic insulin resistance
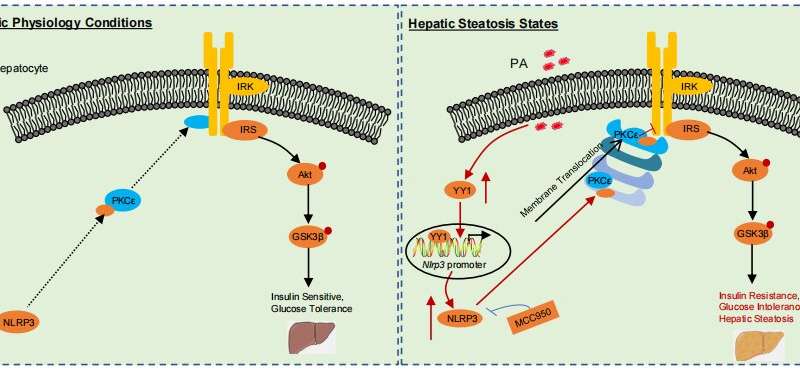
First, Weiwei Qin et al. identified hepatocyte NLRP3 as a crucial inducer of hepatic IR by undertaking multilayer transcriptomic searches and further confirmed that its expression was increased in the liver tissues from NAFLD patients and mouse models (high-fat diet (HFD), leptin-receptor-deficient (db/db) mice), and in palmitic acid (PA)-induced hepatocytes. Subsequently, in vivo studies indicated that loss- or gain-of-function of hepatocyte-specific NLRP3 in HFD-induced mice ameliorated or exacerbated hepatic IR and steatosis, respectively.
Mechanistically, NLRP3 directly bound to and promoted protein kinase C epsilon (PKCε) activation to impair insulin signaling and increase liver steatosis, while inhibition of PKCεdampened the beneficial effects seen in HFD-induced NLRP3-deficient mice. Moreover, we performed screening and discovered that the transcription factor Yin Yang 1 (YY1) positively controlled NLRP3 expression.
These findings prompted scientists to explore therapeutic potential, adeno-associated virus serotype 8 (AAV8)-mediated NLRP3 knockdown in the liver alleviated hepatic IR and steatosis in db/db mice, and pharmacological inhibition of NLRP3 markedly alleviated diet-induced metabolic disorders.
This team identifies a novel mechanism by which NLRP3 drives hepatic IR and steatosis. This finding reveals a previously-unexpected regulatory axis from YY1 to PKCε via NLRP3 induction for metabolic diseases and establishes the YY1-NLRP3-PKCε axis as a potential therapeutic target for NAFLD.
The paper is published in the journal Science Bulletin.
More information:
Weiwei Qin et al, Hepatocyte NLRP3 interacts with PKCε to drive hepatic insulin resistance and steatosis, Science Bulletin (2023). DOI: 10.1016/j.scib.2023.06.003
Provided by Science China Press