Exploiting the cavity-controlled principle in zeolite-catalyzed methanol-to-olefins reaction
Methanol-to-olefins (MTO) process, an innovative and efficient route for olefin production via non-petrochemical resources, has achieved successful development and application in industry and attracted attentions of C1 chemistry and zeolite catalysis in fundamental research.
Dalian Institute of Chemical Physics (DICP) developed DMTO technology that can produce olefins from coal through methanol, which has achieved considerable success in economic income and technological innovation, launching a new era of sustainable olefin manufacturing from non-oil resources.
Since then, DICP has created the second and third generations of the DMTO process (DMTO-II and DMTO-III), which are becoming the important routes for the production of ethene and propene in China. In order to maintain the competitiveness and sustainability of the emerging coal chemical industry, the comprehensive and in-depth understanding of the fundamentals and selective control principles of the catalytic reaction process must be continually deepened to support the development of new catalyst materials and process technique.
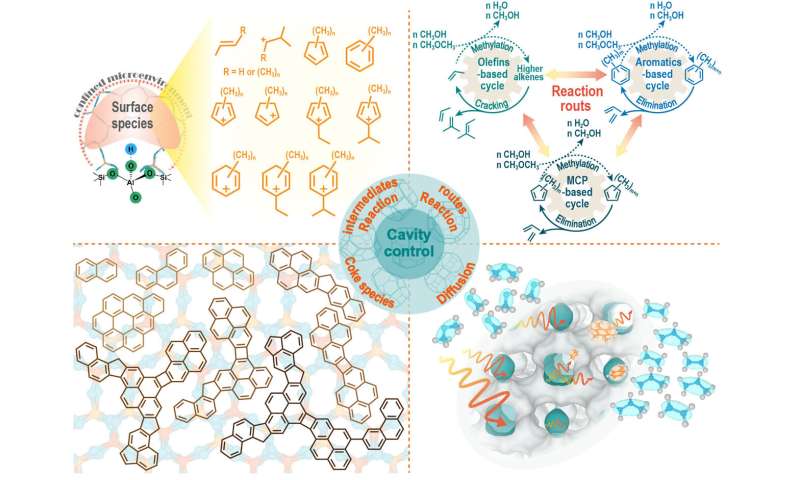
Molecular sieve catalysts, especially for the cavity-type zeolite with small-pore opening, the complex microenvironment embedding the cavity-type structure have exhibited demonstrable features and advantages in shape selectivity of MTO reaction. This complex catalytic environment caused great differences in product distribution, catalyst deactivation and molecular diffusion, revealing the cavity-controlled methanol conversion over eight-membered ring (8-MR) and cavity-type zeolite catalyst.
In the recent review published in National Science Review, the research team led by Profs. Liu Zhongmin and Wei Yingxu (from National Engineering Research Center of Lower-Carbon Catalysis Technology, DICP, CAS) summarized the cavity-controlled principle in the methanol-to-olefins reaction.
Cavity-controlled methanol conversion reaction behavior, cavity-controlled formation of the hydrocarbon pool species and reaction pathway, cavity-controlled catalyst deactivation and diffusion behavior, and the inspired controlled strategies are reviewed as follows.
Cavity-controlled MTO reaction behavior:
Cavity structure and size directly control product distribution, catalyst deactivation and molecular diffusion. The authors reviewed the differences in reaction behavior and product distribution in methanol conversion catalyzed by typical 8-MR and cavity-type zeolite catalysts with similar pore size but different cavity structure. Understanding cavity-controlled MTO reaction behavior would contribute to the establishments of shape selectivity of zeolite materials.
Cavity-controlled reaction intermediates and reaction routes:
The special catalytic microenvironment of cavity-type zeolite varies the reaction intermediates and reaction pathways in the MTO reaction process. This special catalytic microenvironment drives the dynamic evolution of the MTO reaction. The authors expounded the cavity-controlled effect from the generation of hydrocarbon pool species and the dominant reaction path of olefin generation in the complex reaction network.
Cavity-controlled coke formation and catalyst deactivation:
The authors summarized the coke species deposition and deactivation mode in SAPO-34, including the discovery of low-temperature adamantane species, the identification of key precursors during the evolution of polymethylbenzene to polymethylnaphthalene, and the proposed mechanism of cage-passing growth mode of polycyclic aromatic hydrocarbons. Then, the differences in coke species and deactivation mechanisms of zeolite catalysts with different cavity structure were discussed.
Cavity-controlled diffusion:
The authors described the diffusion behavior of cavity-type zeolite, correlating the role of cavity structure and pore size in diffusion, and then revealed the diffusion mechanism of cavity-type zeolite. In addition, the review summarized the diffusion of molecules in acidic cavity with confined organic species, and points out the necessity of coupling diffusion and reaction studies.
Finally, combined with the multiscale and dynamic properties of reaction and catalytic materials in the MTO reaction, the authors proposed the cross-talk mechanism of catalyst material (coke)-reaction-diffusion to reveal the real shape-selective catalysis with interactive behaviors and mechanism in cavity-type zeolite catalyzed MTO reactions.
They pointed out that the key to developing shape-selective catalysts and achieving an efficient process is to establish the best spatiotemporal coordination in the reaction system through mutual echo, mutual modification and mutual guidance among catalyst materials (modified by coke evolution), reaction, and diffusion.
Based on the understanding of cavity-controlled principle, a variety of control strategies, such as modification of the cavity and acidity of zeolite catalysts, pre-coking of fresh catalyst and partial regeneration of the coked catalyst were summarized. In the future, the technical innovation of the MTO process will require accurate control of reaction, coke formation and diffusion in the confined cavity microenvironment to achieve enhanced catalyst stability and product selectivity in the industrial process.
More information:
Wenna Zhang et al, Cavity-controlled methanol conversion over zeolite catalysts, National Science Review (2023). DOI: 10.1093/nsr/nwad120
Provided by Science China Press