Multiomics reveal human umbilical cord mesenchymal stem cells improving acute lung injury via the lung-gut axis
Acute lung injury (ALI) and its final severe stage, acute respiratory distress syndrome, are associated with high morbidity and mortality rates in patients due to the lack of effective specific treatments.
Gut microbiota homeostasis, including that in ALI, is important for human health. Evidence suggests that the gut microbiota improves lung injury through the lung-gut axis. Human umbilical cord mesenchymal cells (HUC-MSCs) have attractive prospects for ALI treatment. This study hypothesized that HUC-MSCs improve ALI via the lung-gut microflora.
Aim
To explore the effects of HUC-MSCs on lipopolysaccharide (LPS)-induced ALI in mice and the involvement of the lung-gut axis in this process.
Methods
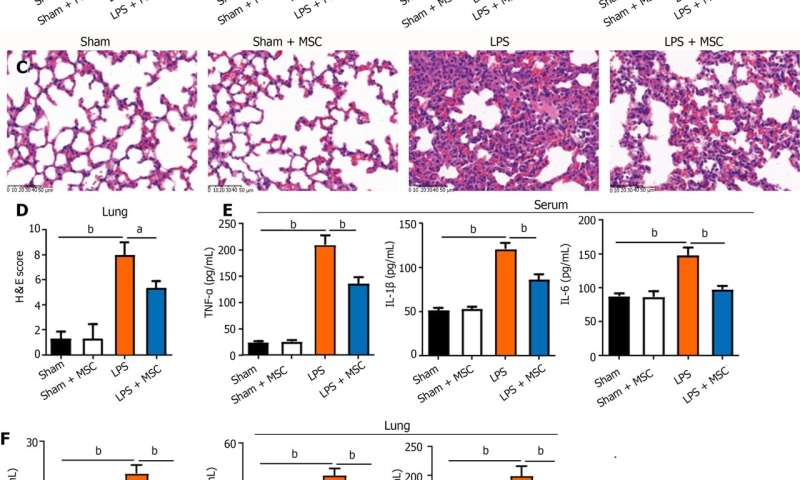
C57BL/6 mice were randomly divided into four groups (18 rats per group): Sham, sham + HUC-MSCs, LPS, and LPS + HUC-MSCs. ALI was induced in mice by intraperitoneal injections of LPS (10 mg/kg). After 6 h, mice were intervened with 0.5 mL phosphate buffered saline (PBS) containing 1 × 106 HUC-MSCs by intraperitoneal injections.
For the negative control, 100 mL 0.9% NaCl and 0.5 mL PBS were used. Bronchoalveolar lavage fluid (BALF) was obtained from anesthetized mice, and their blood, lungs, ileum, and feces were obtained by an aseptic technique following CO2 euthanasia. Wright's staining, enzyme-linked immunosorbent assay, hematoxylin-eosin staining,
Evans blue dye leakage assay, immunohistochemistry, fluorescence in situ hybridization, western blot, 16S rDNA sequencing, and non-targeted metabolomics were used to observe the effect of HUC-MSCs on ALI mice, and the involvement of the lung-gut axis in this process was explored. One-way analysis of variance with post-hoc Tukey's test, independent-sample Student's t-test, Wilcoxon rank-sum test, and Pearson correlation analysis were used for statistical analyses.
Results
HUC-MSCs were observed to improve pulmonary edema and lung and ileal injury, and decrease mononuclear cell and neutrophil counts, protein concentrations in BALF and inflammatory cytokine levels in the serum, lung, and ileum of ALI mice.
Especially, HUC-MSCs decreased Evans blue concentration and Toll-like receptor 4, myeloid differentiation factor 88, p-nuclear factor kappa-B (NF-κB)/NF-κB, and p-inhibitor α of NF-κB (p-IκBα)/IκBα expression levels in the lung, and raised the pulmonary vascular endothelial-cadherin, zonula occludens-1 (ZO-1), and occludin levels and ileal ZO-1, claudin-1, and occludin expression levels.
HUC-MSCs improved gut and BALF microbial homeostases. The number of pathogenic bacteria decreased in the BALF of ALI mice treated with HUC-MSCs. Concurrently, the abundances of Oscillospira and Coprococcus in the feces of HUS-MSC-treated ALI mice were significantly increased.
In addition, Lactobacillus, Bacteroides, and unidentified_Rikenellaceae genera appeared in both feces and BALF. Moreover, this study performed metabolomic analysis on the lung tissue and identified five upregulated metabolites and 11 downregulated metabolites in the LPS + MSC group compared to the LPS group, which were related to the purine metabolism and the taste transduction signaling pathways. Therefore, an intrinsic link between lung metabolite levels and BALF flora homeostasis was established.
This study suggests that HUM-MSCs attenuate ALI by redefining the gut and lung microbiota.
The paper is published in the World Journal of Stem Cells.
More information:
Lu Lv et al, Multiomics reveal human umbilical cord mesenchymal stem cells improving acute lung injury via the lung-gut axis, World Journal of Stem Cells (2023). DOI: 10.4252/wjsc.v15.i9.908
Provided by World Journal of Stem Cells