Hydrothermal synthesis of bifunctional COFs for co-electrolysis of carbon dioxide and methanol
The massive consumption of fossil fuels all over the world has led to excessive CO2 emissions into atmosphere, which has caused serious environmental issues and energy crisis. Electrochemical CO2 reduction (ECR) by renewable electric energy offers a promising strategy to convert CO2 into useful energy substances, which will simultaneously reduce CO2 and produce useful energy fuels.
In recent years, multifarious ECR catalysts have been developed for CO2 reduction, and high efficiencies have also been achieved. However, most studies only focused on the ECR half reaction at cathode, while neglecting the relevant oxidation half reaction in anode side, thus caused a great waste of energy.
In most of the reported research, the conventional method to treat anode reaction was coupled with the water oxidation reactions (oxygen evolution reaction, OER). Unfortunately, this OER process will cause very large overpotential and also need high energy input due to slow kinetics and unfavorable thermodynamics of H2O oxidation reaction.
Additionally, the produced O2 is relative less value-added compared to many industrial chemicals. Therefore, it is necessary to develop an oxidation reaction with high energy efficiency to replace the OER process.
The application of the anodic oxidation process to the organic molecules oxidative synthesis such as methanol oxidation reaction (MOR) to produce HCOOH can effectively improve energy efficiency due to low theoretical overpotential. However, it remains a great challenge to enable these two electrocatalytic reactions to effective cooperate. The main barrier in this field is the lack of highly active electrocatalysts to fulfill these two processes.
Theoretically, the electrocatalysts for ECR and MOR should satisfy the following requirements: (1) highly active and accessible catalytic sites for reduction or oxidation reaction; (2) affinity and adsorption activation for substrates such as CO2 or methanol; (3) preferable electron and proton transfer ability; (4) high stability during the electrochemical measurements. The construction of bifunctional heterogeneous catalysts can effectively solve the above problems which is used for ECR and MOR simultaneously, yet rarely been studied.
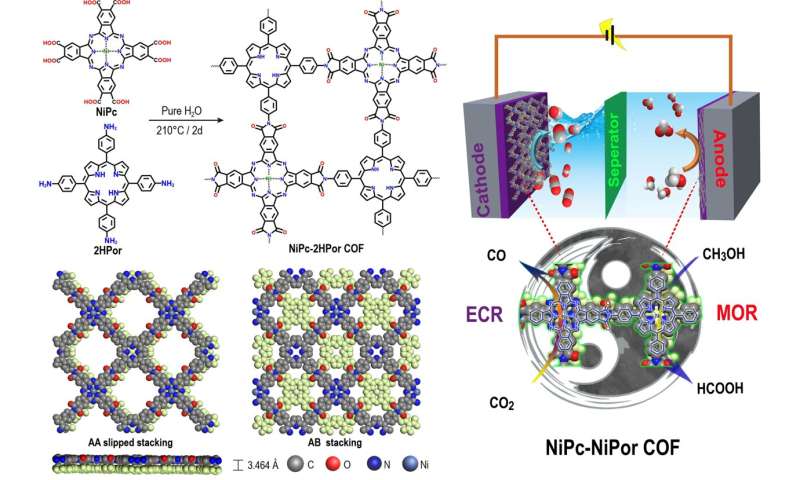
Covalent organic frameworks (COFs) with excellent structural designability are promising platforms for catalytic reactions. Some building blocks of COFs possess appropriate coordination sites, thus making them capable of introducing metal active sites for typical catalysis. Recently, metallophthalocyanine (MPc) and metalloporphyrin (MPor) based-COFs has been studied for catalytic reactions.
Nevertheless, most of works only focused on studying the catalytic performance of single functional component, while the integration of MPc and MPor together into COFs for bifunctional catalysts was still unexplored. Besides, as one of the most important classes of crystalline COFs, Pc-based COFs possess excellent conductivity, mechanical performance and redox-active properties.
However, the traditional synthesis of Pc-based COFs based on solvothermal methods are inevitable to use toxic organic solvent and catalyst. Therefore, it is urgent to develop green and efficient methods to synthesize Pc-based COFs.
On the basis of the above research results, Lan et al. rationally prepared NiPc-2HPor COF by condensing phthalic acid group of NiPc and aromatic amine group of 2HPor through hydrothermal method, and further synthesized NiPc-NiPor COF by post-synthesis coordination reaction. The formed polyimides-linked COFs (PI-COFs) showed high chemical stability and activity for electrocatalysis MOR and ECR.
Above all, the synthesized NiPc-MPor COFs combine the features of crystalline and conductivity, also have multiple active sites with different chemical environment for ECR and MOR. Among them, the NiPc-NiPor COF shows the excellent activity for cathodic ECR and anodic MOR to HCOOH and exhibits remarkable long-term stability.
The in-situ fourier transform infrared spectroscopy (FT-IR) was used to identify the key intermediates for both ECR and MOR. Furthermore, the density functional theory (DFT) calculations demonstrate that the ECR process mainly performs on NiPc unit with the assistance of NiPor, meanwhile, the MOR process more prefers on NiPor and conjugates with NiPc.
The synergistic catalytic effect of NiPc and NiPor together contributes to such high catalytic activity. This is the first report of bifunctional MPc-MPor-based COFs for electrocatalytic cathodic ECR and anodic MOR simultaneously, and it is also of great significance in the field of bifunctional electrocatalysts.
The findings are published in the National Science Review.
More information:
Mi Zhang et al, Green synthesis of bifunctional phthalocyanine-porphyrin cofs in water for efficient electrocatalytic CO2 reduction coupled with methanol oxidation, National Science Review (2023). DOI: 10.1093/nsr/nwad226
Provided by Science China Press