Silica films mean better catalysts in confined two-dimensional spaces
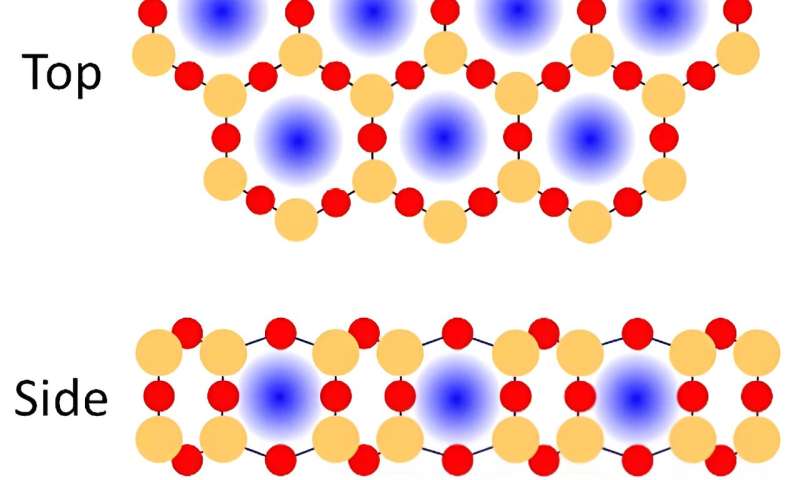
Researchers are making catalysts more efficient by designing materials that contain tiny amounts of catalysts in microscopic cavities. These nanoscale materials have spaces smaller than a single virus. Now, scientists have shown that porous silica films less than one nanometer thick boost the catalytic activity of a metal palladium (Pd) surface for carbon monoxide (CO) oxidation. CO oxidation is a critical reaction in environmental chemistry. The confined two-dimensional space between the metal catalyst and the silica film enhanced CO conversion. It also increased carbon dioxide (CO2) production by 12%, compared to palladium alone.
The paper is published in the journal Angewandte Chemie.
CO can disrupt catalytic reactions and inhibit the desired chemical transformation. Carbon monoxide oxidation converts the detrimental CO to (CO2), typically with the aid of a metal catalyst such as Pd. The converted (CO2) molecule can then easily leave the catalyst surface to be captured or used for other purposes. These results reveal the ways that 2D nanoporous silica films can enhance the oxidation of CO. This approach can be applied to accelerating other types of reactions.
Confined microenvironments have gained attention in catalysis as they can be used to control reaction chemistry. The formation of a 2D nanoscale space between a nanoporous silica bilayer material and a metal catalyst can promote varying kinetic and energetic schemes based on molecular level confinement effects from this reduced volume.
The team placed the bilayer silica atop catalytically active Pd to observe the CO oxidation reaction. The researchers characterized structure-activity relationship with in situ infrared and X-ray spectroscopy and mass spectrometry methods. In situ infrared spectroscopy was used to study the structure of the silica film and identify the binding site geometry of the CO on the metal surface. X-ray spectroscopy identified the oxidation state of the surface and tracked gas evolution at the surface during the reaction.
This work shows that CO oxidation on Pd benefits from confinement effects imposed on surface adsorbates under 2D silica. This interaction results in the formation of a reactive surface oxide that produces 12% higher CO2 formation rates than Pd alone.
More information:
Calley N. Eads et al, Enhanced Catalysis under 2D Silica: A CO Oxidation Study, Angewandte Chemie (2021). DOI: 10.1002/ange.202013801
Provided by US Department of Energy