Scientists continue inquiries into hydrate-based methane storage technology
A paper in Journal of Energy Storage discusses a new biodegradable compound for hydrate formation.
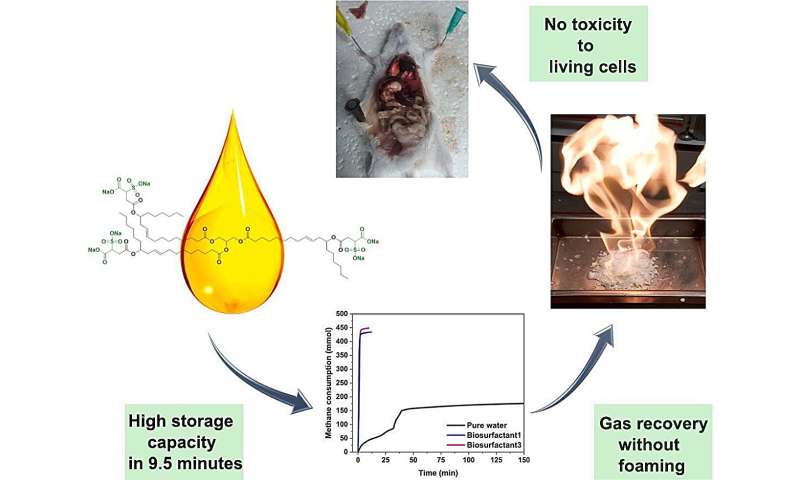
The Laboratory of Enhanced Oil Recovery researches a new class of compounds where castor oil is used as a hydrate formation promoter.
Senior Research Associate Matvei Semenov comments, "Special emphasis is placed on the study of toxicity and biodegradation. This is due to the fact that hydrate formation promoters are designed for hydrate technologies, which in turn are promising for the Arctic regions, where there are strict requirements for the reagents used."
Hydrate-based storage and transportation methods are beneficial because of lenient requirements to temperature and pressure, as well as environmental safety.
A previous study was dedicated to a reagent based on sulfonated castor oil. This time, two sulfated groups were added to a molecule, and the new molecule showed a number of advantages in comparison with a monosulfated analogue.
"Our development allows us to significantly increase the process rate and the overall conversion of gas into hydrate form, and by these indicators we surpass one of the most effective of the existing promoters. In addition, the proposed reagent is devoid of technological disadvantages that many anionic surfactants have. Further development of hydrate technology for storage and transportation of natural gas may become the beginning of a new direction in low-tonnage chemistry and will expand the possibilities of gasification of remote settlements, including those in the Arctic region," notes Head of Enhanced Oil Recovery at KFU's Center for Liquid Hydrocarbons Mikhail Varfolomeev.
"We are in favor of an environmentally safe process—that is, for the cases where our reagent gets into the environment. After all, it is based on natural components. This is an important advantage of our work," adds Lead Research Associate Roman Pavelyev.
The reserves of known hydrate-form extractable resources are higher than existing reserves of standard hydrocarbons. That's why hydrate research is critically important for the future of the energy industry.
More information:
Enhanced methane storage capacity in clathrate hydrate induced by novel biosurfactants: Kinetics, stability, in vivo, and biodegradation investigations
www.sciencedirect.com/science/ … ii/S2352152X23021990
Provided by Kazan Federal University