Molecular mechanism governing RNA-binding property of mammalian TRIM71 protein
TRIM71, also known as Lin-41, belongs to the TRIM-NHL RNA-binding ubiquitin ligase protein family. It was first discovered as a heterochronic gene regulated by microRNA (miRNA) let-7 during C. elegans development and was later shown to play essential roles in the development of mouse and zebrafish.
Subsequently, numerous functions of TRIM71 have been revealed. It regulates the cell cycle progress by repressing the expression of cyclin-dependent kinase Cdkn1a/p21 through the Ago2 and miRNA pathway; it regulates the expression of several pro-differentiation genes in human pluripotent stem cells and promotes reprogramming.
In addition, TRIM71 has been reported to regulate various RNA pathways. In a previous report, the crystal structure of the filamin-NHL domain of zebrafish TRIM71 (DrLin41) complexed with an mRNA stem-loop confirmed that the NHL domain is sufficient for RNA-binding of DrLin41. However, the RNA-binding property of mammalian TRIM71 remains to be elucidated.
A long noncoding RNA (lncRNA) Trincr1 was reported previously and interacts with mouse TRIM71 (mTRIM71) to repress FGF/ERK pathway in mESCs.
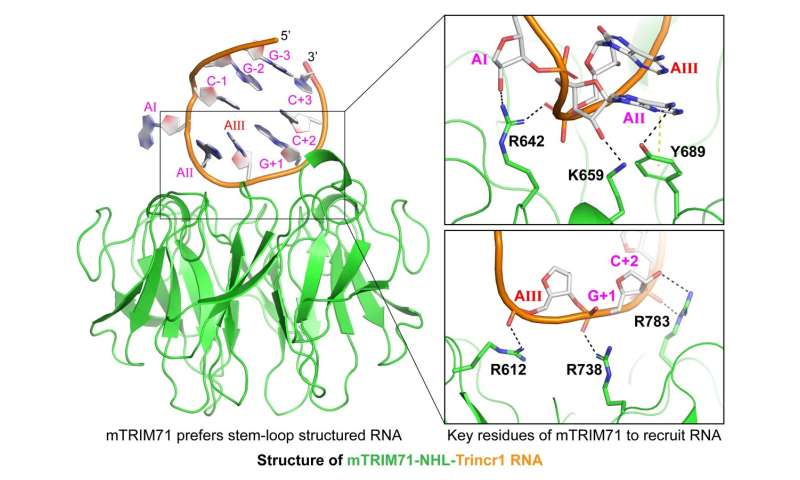
A study led by Prof. Na Yang (State Key Laboratory of Medicinal Chemical Biology, College of Pharmacy, Nankai University) and Prof. Yangming Wang (Institute of Molecular Medicine, College of Future Technology, Peking University) now identifies an RNA motif specifically recognized by mTRIM71 from lncRNA Trincr1, and solves the crystal structure of the NHL domain of mTRIM71 complexed with the RNA motif. The paper is published in the journal Science Bulletin.
From the complex structure, key residues of mTRIM71 are identified, as well as structural and sequential characteristics of RNA that are critical for the interaction between TRIM71 and RNA substrates. The study shows similar hairpin RNAs that TRIM71 prefers to bind exist in the 3'UTRs of Cdkn1a/p21 and Rbl2/p130 mRNA, and mutation of key residues in TRIM71 abrogates its binding to hairpin RNAs in vitro and Cdkn1a and Rbl2 mRNAs in mouse ES cells. In addition, Congenital Hydrocephalus (CH) specific mutation of mTRIM71 impairs its binding to the RNA targets.
In summary, TRIM71 prefers stem-loop structured RNA with specific nucleotide sequence, and single-site mutation of key residues significantly impairs the binding of TRIM71 to hairpin RNAs in vitro and to mRNAs of Cdkn1a/p21 and Rbl2/p130 in mESCs. These results reveal molecular mechanism behind the recognition of RNA by mammalian TRIM71 and provide insights into TRIM71-related diseases.
More information:
Fandi Shi et al, Molecular mechanism governing RNA-binding property of mammalian TRIM71 protein, Science Bulletin (2023). DOI: 10.1016/j.scib.2023.11.041
Provided by Science China Press