Nickel-modified indium with inherent oxygen vacancies for carbon dioxide hydrogenation to methanol
In a study led by Prof. Peng Gao and Prof. Shenggang Li (CAS Key Laboratory of Low-Carbon Conversion Science and Engineering, Shanghai Advanced Research Institute), it was found that the hydrogenation metals (Co, Ni, and Cu) promoted In2O3 catalysts with a similar loading of 1 wt.% were prepared by the hydrothermal method.
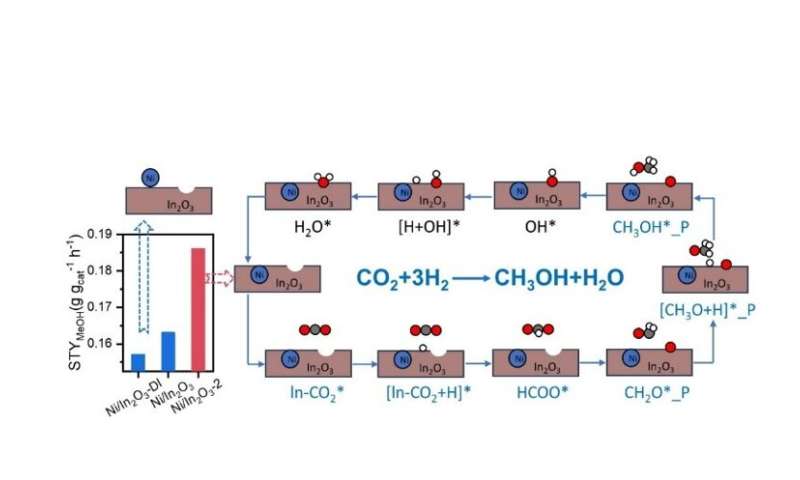
It was found that the Ni-promoted In2O3 catalyst with high dispersion possesses the largest amount of oxygen vacancies and the strongest ability for H2 activation and exhibited the highest CO2 conversion and STY of CH3OH, which reached 0.390 gMeOH gcat−1 h−1 with CH3OH selectivity of 68.7%.
In addition, the Ni-promoted In2O3 catalyst exhibits very stable performance over 120 h on stream, which suggests a promising prospect for industrial applications. Moreover, a series of Ni-modified In2O3 catalysts with different surface Ni contents were prepared to further investigate the effect of oxygen vacancy properties on the catalytic behavior of CO2 hydrogenation to methanol.
Surface Ni doping was found to promote the formation of oxygen defects on the In2O3 surface, resulting in higher CO2 reactivity, whereas the Ni-promoted In2O3 catalyst with more subsurface Ni showed higher methanol selectivity and productivity. The catalytic performance of our Ni/In2O3 catalysts was further rationalized by DFT calculations and microkinetic simulations.
DFT-based microkinetic simulations show that CO formation is preferred at the oxygen vacancy site on the surface-doped Ni/In2O3 catalyst, whereas CH3OH formation is favored at that on the subsurface-doped Ni/In2O3 catalyst especially at relatively low reaction temperatures.
This work thus provides theoretical guidance for improving the CO2 reactivity of In2O3-based catalysts while maintaining high methanol selectivity.
This study not only provides a better understanding of the effect of transition-metal promoters with high dispersion on the catalytic performance but also suggests that DFT-based microkinetic simulations can provide reliable prediction on the catalytic activity and product selectivity for the CO2 hydrogenation to methanol reaction over industrially relevant metal-promoted oxide catalysts, which is crucial for their computer-aided rational design.
The findings are published in the journal Science China Chemistry.
More information:
Zixuan Zhou et al, Nickel-modified In2O3 with inherent oxygen vacancies for CO2 hydrogenation to methanol, Science China Chemistry (2024). DOI: 10.1007/s11426-023-1929-1
Provided by Science China Press