Developing wound dressings with enzymes embedded into the fabric to fight infections
A public health crisis is looming over nearly every medical facility in the country. Each year, over six million people are affected and over 200,000 people die from it. Treating this threat costs tens of billions of dollars annually. And perhaps the most troubling aspect of this threat is that it's microscopic, making itself known only when it has grown to a critical, troubling mass.
The fight against biofilms may seem like an easy battle on the surface. Infections of the dermis—the inner layer of the skin—can come from burns, surgical incisions, or chronic wounds. A simple antibiotic treatment—a go-to remedy for nearly a century—should resolve the infection or at least reduce it.
But what happens when antibiotics no longer become a viable method to treat infections? And what happens when wound dressings like bandages become infected themselves?
Amber Doiron, assistant professor in the College of Engineering and Mathematical Sciences' Department of Electric and Biomedical Engineering, who just won a prestigious National Science Foundation (NSF) CAREER Award, is researching innovative methods and materials to combat these problems while also inspiring the next generation of STEM leaders.
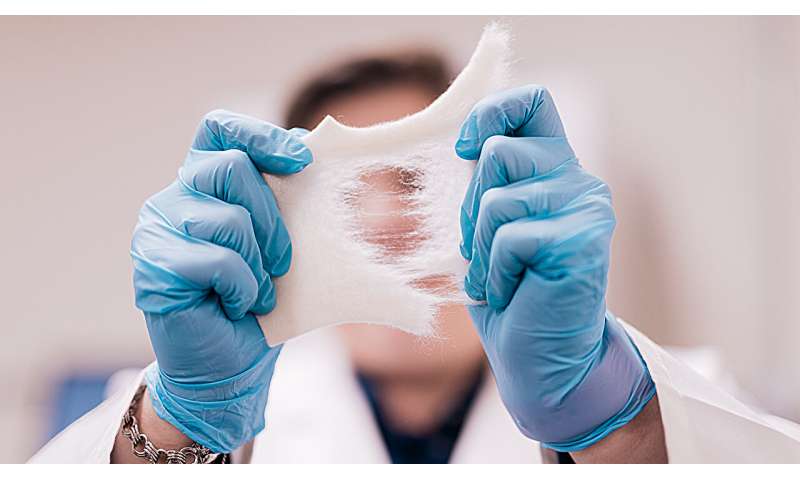
"Our main goal is to invent new materials to act as wound dressings in order to prevent or treat a particularly nasty kind of bacterial infection called a biofilm by depleting a key metabolite," Doiron said. "Getting rid of the metabolite is essentially getting rid of an energy source for the bacteria." And just as everyone needs to eat to gain energy, microscopic bacteria inside biofilms feed on even smaller molecules, namely pyruvate, for their energy.
"If we can get rid of this particular molecule, pyruvate, it tends to prevent these particular types of infections," Doiron said. "We're creating materials that deplete the energy source for the bacteria, which then have a hard time creating the infections in those wounded environments."
Simply, Doiron and her collaborators are creating innovative wound dressings like bandages that make it difficult for infections to turn into biofilms.
"Biofilms occur when bacteria attach to a surface and they create this happy environment for themselves, where they're protected from things like antibiotics or the immune system," Doiron explained. "They become very difficult to treat once the bacteria are attached to the surface and they create this slime-like substance in which bacteria like to live."
While biofilms caused by bacterial growth can affect anyone that has a wound, Doiron explained that there are some populations that are more susceptible.
"Elderly folks, people with medical complications like diabetes. Burns are a classic example," Doiron said. "Any wound that covers a large portion of the body. Anyone that has a wound technically could get a biofilm in it." And since the skin is the largest organ of the body, it's easy to imagine why biofilms are so prevalent.
To fight biofilms, Doiron and her team are making bandages out of clinically-proven materials.
"We're actually basing our design on a material that's already used for clinically and commercially available wound dressings: alginate. It's a natural material harvested from seaweed reformulated in a way that provides wounds with an optimal healing environment," Doiron said. "It stays moist, which allows for the body to go through its normal wound healing process."
But a new wound dressing or bandage isn't enough to completely eradicate biofilm. The bacteria themselves need to be cut off from its source of energy.

"Now we're trying to make some chemical adaptations to that alginate to include an enzyme that is then able to deplete that metabolite and treat the infection," Doiron said. There are some limitations to the enzyme Doiron and her team are using, though, including its cost and chemical challenges.
"Right now we're just working with one single enzyme, but part of the funded work is to investigate whether we can get around having to use that particular enzyme, which is pretty expensive," Doiron said. "We just purchase it and it tends to be pretty finicky. It's really sensitive to temperature and pH changes, like you would see within a healing wound."
Ultimately, Doiron wants to create a clinical, shelf-stable product that could be widely available for whenever a doctor needs it.
"Our ultimate goal is to be able to make this into an actual clinical product. But in that case, it may have to sit on a shelf for months or years," Doiron said. "Inherent in this design is needing to make cost effective choices that are going to be stable over time, at different temperatures, and in different conditions. We're making our chemistry-driven choices based on that final product in mind."
Another vital component of Doiron's CAREER award revolves around her efforts to inform and inspire the next generation of STEM leaders.
"What's inherent in what we're doing in making this material is going through a design process: building, testing, redesigning. That process itself is called the engineering design process," Doiron explained. "Teaching our undergraduates how to do this is certainly a huge part of our curriculum in college, but we'd like to introduce younger students to this mode of thinking."
Doiron works with an organization called Vermont Afterschool—a statewide nonprofit that provides afterschool, summer, and third space learning opportunities during out-of-school hours—and their "Linking Engineering to Life" program, which is aimed specifically at girls and non-binary youth who have historically been underrepresented in STEM fields.
"We introduce the youth to the engineering design process and they design all kinds of things," Doiron said. "They look at building water filtration systems, better coating for drug molecules, prosthetics. They're mocking all these up with cardboard and art supplies."
Doiron is by no means alone in her endeavor to help rid of the world infections and biofilms. She has a team of collaborators both on and off campus, including Dr. Rachael Floreani, an associate professor in CEMS and the director of UVM's Engineered Biomaterials Research Laboratory, Dr. Karin Sauer, a professor of Biological Sciences at Binghamton University, and the many undergraduate and graduate students who study with and learn from her work.
Provided by University of Vermont