CRISPR/Cas9 system enhances disease resistance and genetic research in Chrysanthemum breeding
A research team has established a CRISPR/Cas9 gene-editing system for Chrysanthemum morifolium, targeting the CmPDS gene to explore gene functions and enhance breeding. By combining transient and stable transformations, they achieved precise gene knockouts and verified the system's efficiency with the CmTGA1 gene, linked to Chrysanthemum White Rust (CWR) disease resistance. This effective, heritable CRISPR/Cas9-mediated genome-editing system promises to advance genetic research and improve the disease resistance and breeding of chrysanthemums, paving the way for more robust ornamental plants.
CRISPR/Cas-mediated genome editing is a low-cost, highly efficient technique for creating mutants with complete gene knockouts. Despite CRISPR/Cas9 has been successfully applied to model plants, efficiency varies greatly in different experiments and often results in a high miss rate. In C. morifolium, existing methods like VIGS and RNAi fail to achieve complete knock-out of a target gene.
A research article published in Ornamental Plant Research on 16 May 2024, aims to establish a reliable CRISPR/Cas9 system for chrysanthemum using Golden Gate Assembly system and transient CRISPR/Cas9 editing in plants (TCEP) method, focusing on enhancing disease resistance by targeting the CmTGA1 gene.
Using the pCBC-DT1T2 plasmid as a template, a 626 bp band was obtained via overlapping PCR and inserted into the BsaI site of pHSE401. The CRISPR-CmPDS knockout vector was transferred into Agrobacterium strain GV3101 via the freeze-thaw method. Verification of the editing efficiency using RT-qPCR showed a significant reduction in CmPDS expression (19.1%–52%) in transgenic plants.
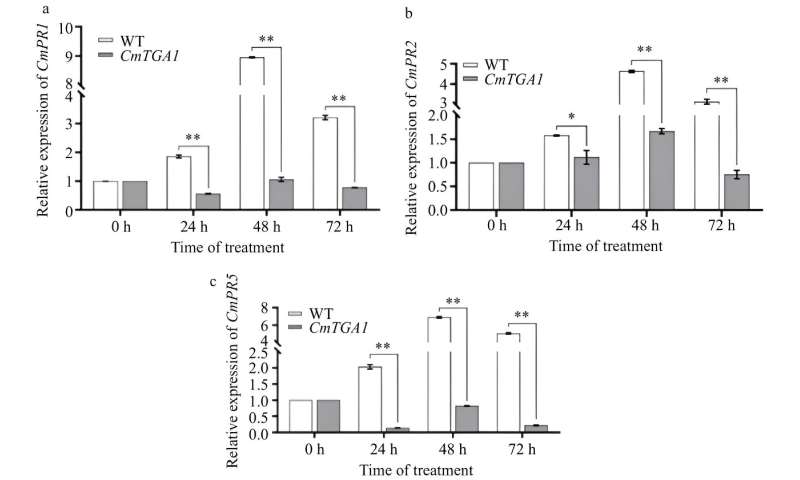
Stable genetic transformation obtained 12 antibiotic-resistant buds, 66.7% of which exhibited albino phenotypes, indicating successful gene knockout. Additionally, a CRISPR-CmTGA1 knockout vector was created and verified for editing efficiency, showing a significant decrease in CmTGA1 expression (20.1%-70.3%).
Stable transformation and sequencing revealed successful gene editing in three stable transgenic plants. Knockout of CmTGA1 resulted in reduced resistance to Puccinia horiana, validating the system's effectiveness in gene editing for disease resistance research in C. morifolium.
According to the study's lead researcher, Hongyu Mao, "In our study, we first targeted knockout of the CmPDS gene in C. morifolium and verified editing efficiency by knocking out CmTGA1. Knockout of the CmPDS gene provides a clear visible marker of transformation. The successful knockout of CmTGA1 further supports the reliability of our system and provides important information for battling CWR disease."
In summary, these findings demonstrate the effectiveness of combining transient and stable transformations for reliable gene editing in chrysanthemums, paving the way for future genetic improvements and enhanced disease resistance in ornamental plants. Future research will focus on optimizing this gene-editing platform and exploring its applications in creating new disease-resistant and stress-tolerant chrysanthemum varieties.
More information:
Qi Chen et al, Establishment of a CRISPR/Cas9 gene-editing system for Chrysanthemum morifolium, Ornamental Plant Research (2024). DOI: 10.48130/opr-0024-0012
Provided by Maximum Academic Press