4-D nanoparticles open new perspectives in safer treatment of tumors with nanomedicine
In the context of nanomedicine, nanoparticles are effective because they can be endowed with multiple functions and are able to hit their target without the need for extremely high doses, which are associated with dangerous side effects. However, they tend to remain in the body for an indefinite time, with important risks to the health of patients. Ideally, nanomedicines should behave like a 4-D material, developing nanoparticles for diagnosis (for example by magnetic resonance imaging or CT scan) and cancer therapy that have as their main requirement the ability to biodegrade, not to accumulate in the body, limiting it in this way the side effects.
We published an article in ACS Nano that explains how inorganic nanoparticles based on an alloy of gold and iron, two biocompatible elements, which are therefore particularly suitable for applications in the biomedical field, are able to biodegrade spontaneously in living organisms
To understand our study, titled "4-D Multimodal Nanomedicines Made of Nonequilibrium Au-Fe Alloy Nanoparticles," we can use the metaphor of a comet hunter.
Just imagine a comet: a mass of frozen gas that passes close to the Earth at high speed and whose destiny is to gradually evaporate until it disappears. On the one hand, it is not easy to capture one; on the other hand, you don't have that much time to observe it as it is in dynamic and rapid transformation. Now, let's think of a "hunter" researcher who has to do with a microscopic comet composed of elements of the periodic table that do not love each other very much—that is, whose destiny is to separate in space and dissolve in biological environments. There is a moment before dissolution when the elements are "trapped" in what is called a nonequilibrium (or "metastable") nanoparticle. Since this metastable nanoparticle changes over time, it is not enough to provide its composition and size at any given time, but one should also know how it might change in the future or how it just mutated. It can therefore be defined as a 4-D nanosystem, where time is added to the three traditional dimensions (height, width and depth). Yes, but in what field would it be useful?
Our research highlights how the possibility of capturing these "4-D nano-comets" is fundamental in the field of nanomedicine, and especially for the diagnosis and treatment of cancer, showing that metastable gold-iron-based nanoalloys could be ideal candidates for the purpose.
What is the discovery about?
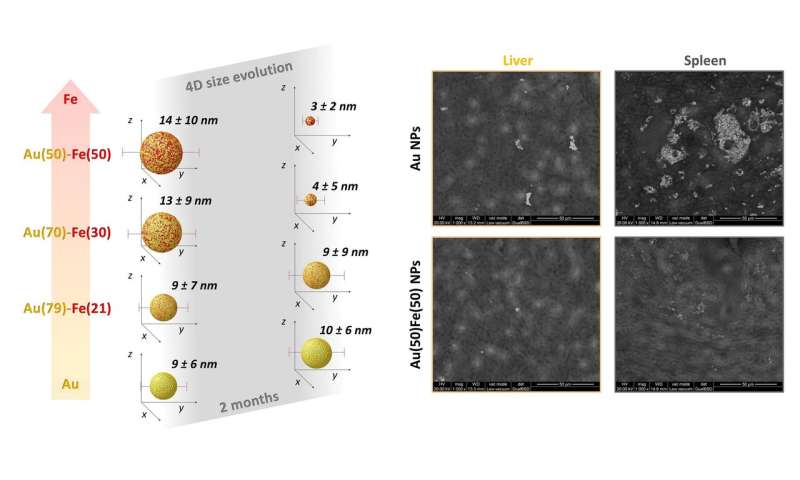
Currently, molecular compounds, not nanomaterials, are not used as contrast agents. For instance, in magnetic resonance imaging, gadolinium chelates are used. However, these can accumulate in the tissues and stimulate immune or allergic responses. They have a limited bio-persistence over time and therefore force operators to the administration of very high doses with possible side effects on the kidneys, where these compounds accumulate mainly in the very first hours after administration. There is an important series of side effects related to the accumulation of molecular contrast agents used in the clinic. For the nanomaterials studied as alternatives, the problem is the opposite, since they tend to accumulate in the body and remain there for an indefinite time. We have focused on 4-D nanomaterials, which have the ability to change shape, size and structure over time and are able to degrade and disappear spontaneously after use. We have experimentally demonstrated that the nanoparticles of gold-iron alloys containing the two elements in "non-equilibrium" proportions possess these characteristics.
What is the main result of the study?
The research, which started with a theoretical-computational investigation, has shown how the atoms of gold and iron, two biocompatible elements that are particularly suitable for applications in the biomedical field, must be arranged inside the nanoparticles so that the latter biodegrade spontaneously in living organisms. The key to the whole study was to find a way to "force" iron and gold to coexist in proportions that are not practicable in nature. For this purpose, laser synthesis techniques in liquid were used to produce bi-metallic Au-Fe nanoparticles capable of biodegradation. These metastable nanoparticles have also been tested in vivo, where they have been shown to leave the organism after a not excessively long period, as opposed to other nanoparticles based only on gold or only on iron oxide, which instead tend to persist for much longer times.
Having a 4-D nanomaterial exploitable as a multimodal imaging agent is particularly important at a clinical level because it allows reducing both the dose administered to the patient and the waiting time before the imaging itself, which are crucial in the treatment of tumors. The next step will be the investigation of the theranostic (i.e., diagnostic and therapeutic) potential of these 4-D nanomedicines.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about ScienceX Dialog and how to participate.
More information: Veronica Torresan et al. 4D Multimodal Nanomedicines Made of Nonequilibrium Au–Fe Alloy Nanoparticles, ACS Nano (2020). DOI: 10.1021/acsnano.0c03614
Veronica Torresan is a PhD student in Materials Science and Engineering at the University of Padova, after having been a research associate in the Laser Assisted Synthesis and Plasmonics laboratory of the same university. She focused her research activity on the synthesis and biomedical applications of inorganic and hybrid materials.
Daniel Forrer is a Researcher at the Institute of Condensed Matter Chemistry and Energy Technologies (ICMATE) of the Italian National Research Council (CNR) and at the Department of Chemical Sciences of the University of Padova. He was visiting fellow at the Scuola Normale Superiore and at the ICCOM in Pisa and visiting research associate at Princeton University. He is an expert in the DFT modeling of inorganic and hybrid materials. His research activity is particularly focused on the thermodynamics of redox processes taking place at metal surfaces and on the structure and reactivity of self-assembled monolayers at inorganic surfaces.
Vincenzo Amendola is associate professor of Physical Chemistry at Padova University, where he obtained the PhD in materials science in 2008 and the Italian qualification as full professor in 2017, after research experience at M.I.T. and Cambridge University. With his Laser Assisted Synthesis and Plasmonics lab, he seeks for unconventional and non-equilibrium nanomaterials exploitable for experimental and theoretical investigations in plasmonics, sensing, nanomedicine and catalysis.