Novel cell engineering method for drug discovery, biologics and cell therapy
Every graduate student, creative person and inventor of any stripe has experienced periods when nothing seems to come together, some of us many times. I was waist-deep in such pit after a year-long effort to make a large number of stable cell lines, which I needed to finish my doctoral studies as a graduate student at The Rockefeller University, failed. I invented Chromovert technology because I had no choice; I simply had to figure out a way to reach my goals better and faster in order to graduate. "Could molecular beacons and fluorescence-activated cell sorting be combined to detect and fish out transfected cells expressing multiple desired genes?" I wondered. They can, and that, in a nutshell, is the Chromovert invention.
Chromovert is an addition to the cell and genetic engineer's toolkit. The technology has been demonstrated for making cell lines and cell-based assays for drug discovery of a broad diversity of challenging ion channel and GPCR drug targets. The largely automated process may be used to implement "drugomics" as a new branch of the -omics sciences for "drug discovery at scale."
For readers of Science X, I offer a practical guide and key insights my team and I gleaned while pioneering the technology, including novel capabilities, technology overview, example results and closing remarks. The citation for the scientific publication is below.
Novel capabilities
We used Chromovert to produce robust stable cell lines for proteins others reported to be cytotoxic or nonfunctional in laboratory cell cultures. Greater study is needed to understand the basis for this enabling capability. My hypothesis, based on our empirical results, is that by using molecular beacons to light up cells capable of high-level expression of target genes while scanning orders or magnitude greater numbers of cells than possible before, Chromovert permits isolation of even exceedingly rare cells for functional, viable and stable expression of otherwise challenging genes. The average laboratory cell may not provide the cellular milieu required for expression of certain proteins, for instance, ion channels or GPCRs that are normally expressed in specialized cells such as neuronal cells or polarized epithelial cells. However, laboratory cell cultures exhibit high levels of cell-to-cell genetic diversity. Chromovert may work by detecting rare cells suitable for expression of the target genes.
Importantly, Chromovert is a platform technology. Like real-time qRT-PCR, the method is based on the two broadly applicable principles of nucleic acid hybridization and fluorescence resonance energy transfer (FRET). Unlike PCR methods, however, Chromovert operates in the context of living cells. Additionally, we have developed novel RNA detection tags termed "Chromo-Tags" that further streamline the method. Cookie-cutter production of cell lines is possible without the need to design and develop any gene-specific probes, as described below.
Anna Nowogrodzki said it best in her review of CRISPR for The Scientist: "Almost always, building something is harder than tearing it down. Similarly, knocking in genes poses a greater challenge than knocking them out." Unlike CRISPR, which is especially well-suited to deleting or disrupting target genes in treated cells, Chromovert is about creating, detecting and isolating cells treated to comprise one or more added genes.
Technology overview
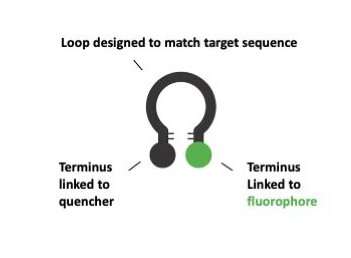
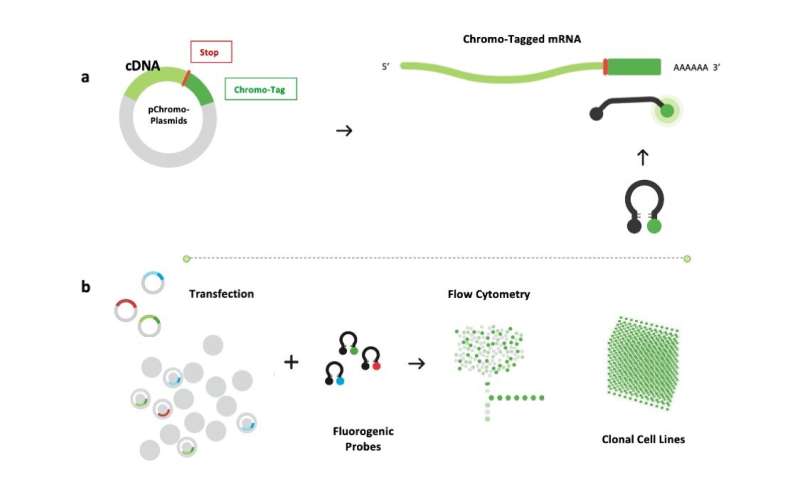
Chromovert is a novel cell engineering method that we developed to detect and isolate individual living cells that comprise one or more desired genetic sequences. The technology uses molecular beacons or fluorogenic oligonucleotide signaling probes directed to target genes. Probes are designed to form stem-loop structures with a fluorophore and quencher paired to absorb its emission covalently attached at either terminus, and an intervening target-specific loop sequence (see Fig. 1).
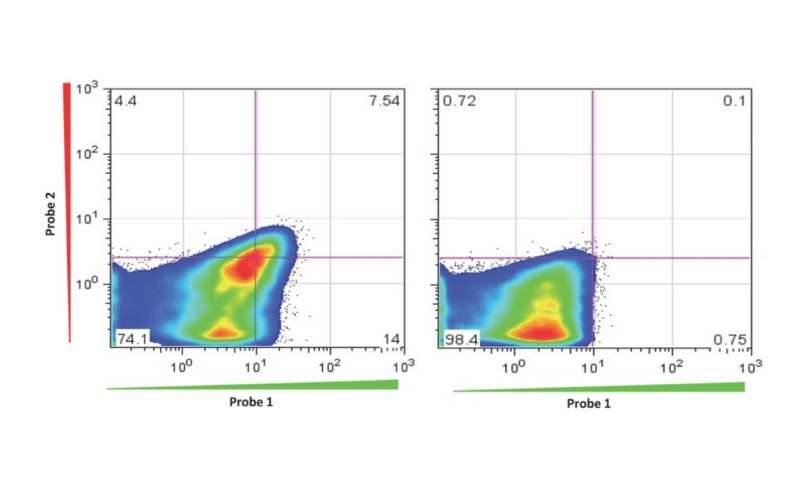
Upon hybridization, probes undergo a fluorogenic conformational change that displaces the fluorophore from the vicinity of the quencher. Positive cells may then be detected and isolated using flow cytometry (see Fig. 2; a technology flowchart is also provided in Fig. 3). Chromo-Tags allow a "plug and play" approach to using Chromovert. Chromo-Tags are 3´ untranslated RNA sequence tags subcloned for expression downstream of the stop codon of cDNAs of interest. Unlike epitope tags that decorate polypeptides at the amino acid level, Chromo-Tags are not translated into protein, such that only native protein sequences are expressed. Chromo-Tags streamline the cell engineering process by obviating the need to design and develop gene-specific probes.
A note for users: During the adaptation of Chromovert to a new cell culture, and when gene-specific probes are being developed, care must be taken to ensure that any fluorescence from cell-surface labeling by aggregates of probe is differentiated from target-dependent signal. Cells transfected with a target gene or control transfected cells and a probe directed to the target sequence and control probes may be used to test for signal over background only in the case of test cells using test probe.
Example results
In the scientific publication of Chromovert technology, we report example results for ion channel and GPCR proteins. Here, I provide some highlight and insights.
First, we found that the creation of a stable multigene cell line can sometimes produce results that are not possible to replicate using transient, inducible or other expression systems. An epithelial sodium channel (ENaC) is an ion channel that is expressed in polarized epithelial cells as well as in human taste receptors. Our study is the first to confirm its role as the human salt taste receptor. We did this by identifying a corresponding potentiator and using human sensory taste testing studies that confirmed its enhancement of the salty taste of table salt. While the proteolytic state of ENaC in the oral cavity has not been defined, we found that partial proteolysis of our ENaC cell line in the context of a cell-based assay was critical to identify salt-taste-enhancing compounds. Standardized conditions for cell-based proteolysis of αβγ-ENaC cannot be approximated using transient transfection systems, inducible expression or expression of the pore-forming α-subunit alone. In this case, the use of Chromovert to enable the production of a functional, viable and stable αβγ-ENaC cell line was just the first step of achieving in vitro correlates for a physiologically relevant form of ENaC as it exists in the body.
Second, in the case of GABAA ion channels, we created a panel of four multigene cell lines that recapitulated gold-standard electrophysiology results, but using much less expensive and HTS-friendly fluorescent membrane potential dyes and cell-based assays. The standardized Chromovert process using Chromo-Tags was used to create the panel. As such, the panel provides proof-of-concept and feasibility data to implement a combinatorial panel of cell lines representing all known and putative combinations of the GABAA subunit genes.
CNS drug targets like GABAA are large gene families that mediate important functions like anxiety, sedation and memory. Many CNS drugs have severe side effects including suicide ideation. As opposed to the traditional approach of creating one cell line expressing one combination of GABAA subunits for HTS, our approach makes it possible to create a combinatorial panel of 10,000+ GABAA cell lines each expressing a different combination of subunits. Known drugs and failed candidate drugs may be tested using the combinatorial panel to correlate specific cell lines and corresponding subunit combinations with desired efficacy and side effects in vivo. This is a functional genomics approach we term "drugomics."
The same approach can be used to tease apart the functional systems-biology of other heteromultimeric ion channels and large gene families. For instance, whereas most drug discovery efforts in cystic fibrosis (CF) focus on the Δ508 mutant of the CFTR gene, which accounts for ~70% of patients, 30% of patients suffer from rare mutations that are not addressed. Chromovert removes the time, cost and effort to implement cell lines and cell-based assays for the remainder of the approximately 400 CFTR mutations implicated in CF. In our study, we provided results for the automated production of robust CFTR and Δ508 CFTR cell lines with greater functionality compared to existing systems, including transient transfection.
Finally, as additionally shown in the case of the GPCR and NaV1.7 examples in our report, Chromovert allows the production of cell lines expressing one or more genes for a broad variety of proteins. All the examples reported in our publication were created using optimized pChromo mammalian expression plasmids that comprise Chromo-Tags. No gene-specific probes were designed or used for any of the reported results. The pChromo plasmids and corresponding fluorogenic oligonucleotide Chromovert probes are available from Secondcell Bio at www.secondcellbio.com/.
Closing thoughts
Chromovert is a platform cell engineering tool that permits the creation, detection and isolation of even exceedingly rare cells treated to comprise one or more added genes. Chromo-Tags are novel RNA detection tags that allow the method to be used without the need to create gene-specific signaling probes. Chromovert has been demonstrated for the production of a broad variety of multigene cell lines.
Currently, the industry average failure rate for drug discovery programs in pharmaceutical companies is reported to be approximately 98%. Although this includes failures at all stages of the process, the high failure rate points to a dire need for any improvements in the efficiency of the process. One factor contributing to the high failure rate is the lack of cells that accurately mimic the biology of human disease in the laboratory dish. Indisputably, drug discovery research would benefit from cells that express drug targets in a form that mimics human disease with high fidelity for use in cell-based assays. Consequently, there is a great need for rapid and effective establishment of cell-based assays for more rapid discovery of new and improved drugs.
Chromovert Technology is designed to fit the need. Starting from genomics almost 20 years ago, the systems-biology approach of the -omics sciences continues to provide rich datasets to identify biological targets for drug discovery, yet the benefit of all this data evaporates during the final step of drug discovery itself, which is often carried out for one drug target at a time. Chromovert permits parallel drug discovery against all drug targets as the crucial final ingredient to discover and develop improved drugs with desired selectivity for safer and more efficacious drugs with fewer side effects.
In addition, Chromovert opens up a number of additional applications from biologics to cell therapy. For instance, Research Foundation to Cure AIDS is researching and developing the method to increase the efficiency of existing cell therapy strategies to cure HIV-1 infection. Many cell therapy strategies have produced promising results in the lab, but they remain far too inefficient for practical use. In these cases, Chromovert has potential to detect and enrich optimally modified cells.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about ScienceX Dialog and how to participate.
More information:
Shekdar, K., Langer, J., Venkatachalan, S. et al. Cell engineering method using fluorogenic oligonucleotide signaling probes and flow cytometry. Biotechnol Lett 43, 949–958 (2021). doi.org/10.1007/s10529-021-03101-5
Kambiz Shekdar obtained his Ph.D. at The Rockefeller University, where he invented the novel cell engineering method Chromovert Technology and established several research organizations based on it, including Chromocell Corporation, Research Foundation to Cure AIDS (RFTCA) and Secondcell Bio.