Drying capillary bridge loaded with colloidal particles offers a tunable mechanism for controlled particles deposition
Have you ever spilled coffee on a solid surface such as the floor, table or clothing? Most of us have had the experience, but if you have not, or if you are curious, spill just a drop and let it dry for some time. You will notice that as time passes, the droplet gradually becomes smaller until it completely evaporates, leaving behind a stain on the surface. This stain contains the residual solid particles present in your drink.
If you observe closely, you will see that most of the particles are deposited near the original periphery of the drop, characterized by a relatively darker stain. What you have witnessed is known as the "coffee-ring effect," and it has been one of the prime focuses of research in the field of colloidal systems and interfaces in the last two decades. Deegan et al. explained the mechanism for this observed behavior based on a relatively higher evaporation rate near the periphery compared to other areas of droplets. Higher evaporation generates an internal flow, pushing particles from bulk toward the boundary, settling them and giving rise to the well-known ring-like deposit.
But why should you care? Since the seminal work of Deegan et al., many researchers have expanded the paradigm by considering parameters such as properties of liquids and particles, ambient conditions and surface nature. The overall objective is to understand the deposits first and then either control the parameters to get desired deposits or predict the evaporation parameters by analyzing the residual deposits. The former has been of interest for applications involving micro/nanopatterning and inkjet printing, while the latter contributes to disease diagnosis, forensics and product quality inspections.
What are we up to?
While droplet deposits are significantly studied, we know little about liquid evaporation in other geometries. In our recent work, we explore one such geometry, the capillary bridge. It forms by entrapping fluid between solid surfaces sufficiently close to each other. We observed the evaporation of liquid suspensions containing colloidal particles between parallel plates. In this case, apart from the parameters mentioned above for droplets, it becomes possible to control the gap between surfaces, the dimensions of plates, and the nature of the two plates. Hence, this particular geometry provides more parameters to control deposits, which are easier to tune than manipulating ambient conditions or properties of liquid and colloidal particles.
To fully understand and characterize the capabilities of this particular technique, we performed experiments using varying particle sizes, concentration and the nature of plates, one at a time. We analyzed the obtained deposits using optical and scanning electron microscopy. We further developed a computational model to better understand the mechanisms at play, which captured our experimental results with reasonable fidelity. To expand the usability of the results, we also theorized a simplistic model including the effects of all relevant parameters, which agrees well with our computational and experimental observation.
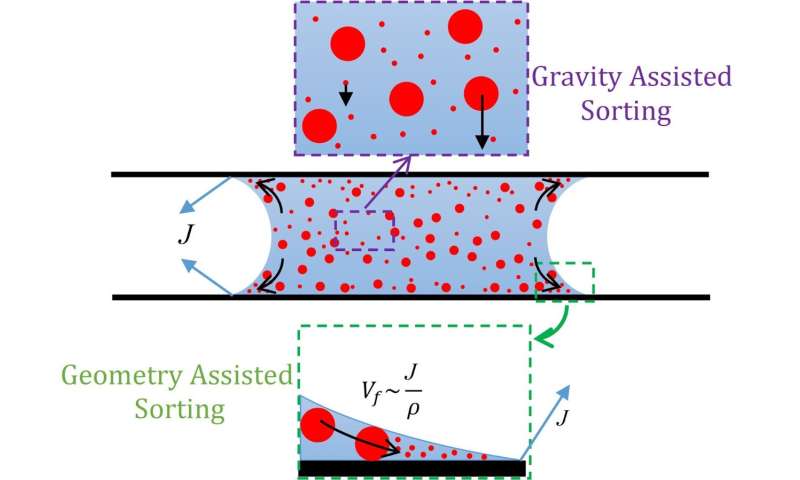
One interesting observation was the preferential deposition of heavier particles on the bottom plate during experimentation with single-size particles. This is because heavier particles can experience more gravitational pull than the forces from internal flow and hence might follow gravity and deposit on the bottom plate. But as we already know, the evaporation rate and subsequently internal flow could be altered based on the parameters as mentioned earlier, providing a mechanism to vary the relative contribution of gravity. This finding inspired us to investigate separating particles of different masses using the capillary bridge. We proved via experimentation that it indeed leads to better segregation than traditional droplets. We highlight two mechanisms, namely gravity-based sorting and geometry-based sorting (Fig. 1), which could be invoked simultaneously to achieve high separation efficiency.
We believe our work could help researchers to design more controlled particle deposition via droplet evaporation than previously possible.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about ScienceX Dialog and how to participate.
More information: Robert D. Deegan et al, Publisher Correction: Capillary flow as the cause of ring stains from dried liquid drops, Nature (2021). DOI: 10.1038/s41586-021-03444-z
Gaurav Upadhyay et al, Colloidal Deposits via Capillary Bridge Evaporation and Particle Sorting Thereof, Langmuir (2021). DOI: 10.1021/acs.langmuir.1c01869
Bio: Gaurav Upadhyay is a Ph.D. student at University of Illinois at Urbana-Champaign. He earned his M.S. degree in Mechanical Engg at Indian Institute of Technology (I.I.T.) Bombay in 2021. Rajneesh Bhardwaj is currently a Professor at Mechanical Engineering Department, I.I.T. Bombay, Mumbai, India. His research interests are in droplets, interfacial flows and fluid-structure interaction.
Laboratory website: sites.google.com/view/interfaces-fsi-lab/home