New cross-electrophile coupling of heteroarenium salts and aryl iodides
Researchers from Auburn University have found a new pathway to use visible blue light to promote nickel catalyzed cross-coupling of activated heteroarenes with aryl iodides. This would be highly valuable to the pharmaceutical and agrochemical industries.
"It was outstanding to see the reaction go from 5 to 87% yield just by using blue LED light with a 1% photocatalyst in 2.5 hours," said Dr. Rajender Nallagonda, one of the first authors of the study. This work was published on January 19th in the journal ACS Catalysis.
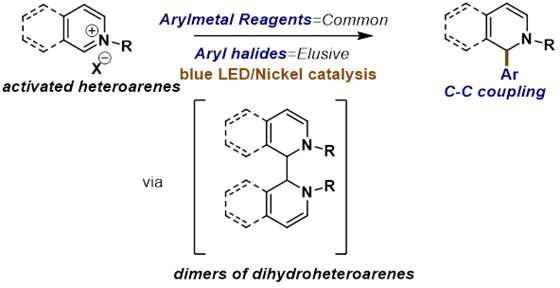
This is the first reported article for the synthesis of dihydropyridines using a light-promoted, cross-electrophile coupling of heteroarenes with aryl iodides using nickel catalysis.
Dihydropyridines molecules appear frequently in drugs and natural products. Most dihydropyridines have been synthesized using traditional nucleophilic addition of organometallic reagents to activated heteroarenes. However, organometallic reagents are more costly, less commercially available, more reactive, and limit the types of molecules that can be made, especially if the reaction is done on a commercial scale. Nallagonda et al. recognized that these organometallic and organoboron reagents are usually prepared from the corresponding aryl halides, which are readily available and less reactive. It would be highly advantageous to substitute aryl halides for the organometallic partner in addition to heteroarenes because it could dramatically increase the number and types of molecules that can be easily accessed. This type of reaction can be termed a "cross-electrophile coupling" reaction.
The challenge of the transformation is that it requires precise control of selectivity for cross-coupling over homocoupling and control of regioselectivity on the heterocycle. Nallagonda et.al found that the blue light was very crucial for this reaction to couple aryl iodides to heteroarenes. To confirm their understanding of how the blue LED light worked together to promote the reaction, Dr. Nallagonda performed a series of mechanistic experiments. He synthesized a dimer of dihydropyridine, which is intermediate in the reaction. He found that without the presence of light, the dimer was unable to couple with aryl iodide to form the product. Further computational studies confirmed that the dimer of dihydropyridine was undergone photoexcitation under blue light, which could transfer electrons to nickel intermediate to form the desired bond. This is a very unique mechanism. "This work opens up many new avenues that could affect many types of transformations," says Professor Rashad Karimov corresponding author of the study.
This is exciting because radical intermediates can be difficult to control in reactions and this offers a new strategy. This new reaction has reduced the number of steps involved in the synthesis of dihydro heteroarenes, which will save pharmaceutical companies time and money.
The researchers found that a wide range of aryl, heteroaryl, and vinyl iodides was viable with this reaction to produce various dihydropyridine derivatives. Amide groups on pyridinium salts play a directing group role to deliver dihydropyridines with vicinal substitution patterns. Besides, a new standard method can aid in adding a variety of functionalized aryl groups to heteroarene salts including functional groups which are not tolerated by organometallic reagents.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about ScienceX Dialog and how to participate.
More information:
Rajender Nallagonda et al, Light-Promoted Dearomative Cross-Coupling of Heteroarenium Salts and Aryl Iodides via Nickel Catalysis, ACS Catalysis (2022). DOI: 10.1021/acscatal.1c05780
Rajender Nallagonda received his Ph.D. degree in Chemistry from the Indian Institute of Science Education and Research Bhopal in 2016 under the supervision of Professor Prasanta Ghorai. Subsequently, he worked at IIT Bombay with Professor Chandra. M. R. Volla, at the HUJI, Israel with Professor Ahmad Masarwa and at the University of Bristol, U.K. with Professor John Bower. In September 2019, he moved to the Karimov group, Auburn University where he works on stereoselective dearomatization of the heteroarenes.
Rashad Karimov received his MS degree from University of Minnesota, Duluth and a PhD degree from Cornell University. He worked as a postdoctoral research fellow at the University of California, Berkeley from 2014–2017 before joining Auburn University in August of 2017. He belongs to several professional societies including American Chemical Society. His current research concerns development of transition metal catalyzed reactions for the synthesis of partially saturated heterocycles.