Developing novel peptide-based chemotherapy using a combination of computer simulations and nanotechnology
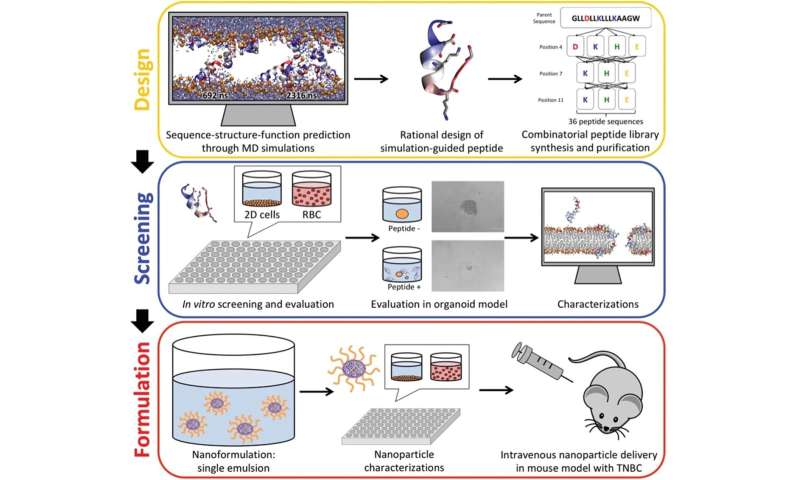
Membrane-active peptides, small proteins composed of less than 50 amino acids that interact with cellular membranes, have been proposed as a promising treatment modality in searching for next-generation chemotherapeutics. Peptides that permeabilize and lyse the target's cell membranes are a ubiquitous part of the innate immune defense and have long been envisioned as therapeutic candidates against pathogens. Against cancer, membrane-active peptides can lead to rapid necrotic cell death of their target cells, and the growing enthusiasm toward these tailorable therapeutic candidates is accompanied by increasingly sophisticated efforts at expanding their synthetic flexibilities and chemical repertoire. Therefore, membrane-active peptides may help address several common shortcomings among standard-of-care small molecule chemotherapeutics targeting biochemical pathways, including drug-resistance development and ineffectiveness against quiescent cancer cells that are frequently associated with treatment failure and tumor relapse.
Although several naturally derived membrane-active peptides, e.g., colistin and daptomycin, are clinically approved as drugs to treat bacterial infections, their vast versatility and diverse range of molecular targets have impeded the development of new and optimized designs. In addition, translational challenges common to most peptide therapeutics, namely toxicity, poor plasma stability, and pharmacokinetics have limited translation of promising candidates.
Developing and applying a combination of computer simulations and nanotechnology, we successfully identified a new route to greatly accelerate the design and characterization of lead compounds and to rationally optimize their pharmaceutical properties against triple-negative breast cancer, a type of breast cancer that lacks the protein receptors commonly found in breast cancer, in xenograft mouse models.
In past decades, molecular dynamics, representing a physical movement simulator of atoms and molecules, have been widely used in drug discovery and protein engineering. This computational tool is useful in studying protein structures and functions and dynamic events.
In this work, we applied simulation-guided rational combinatorial peptide design, synthesized the peptide library, evaluated them against a panel of cancer and non-cancer cell lines, and identified several lead candidates that have significantly favorable activity toward cancer cells through the acidic tumor microenvironment. Remarkably, these peptides are neutral charged at physiological pH conditions in healthy cells but are charged under slightly acidic conditions, typically found in the tumor microenvironment. Using this methodology, we were able to greatly amplify the selectivity of the anticancer peptides, with strong toxicity for cancer cells while leaving healthy cells unharmed.
To demonstrate the translational potential, we utilized state-of-the-art nano-formulation technology to encapsulate the lead anticancer peptide in small polymeric nanoparticles with a size of 20 nm diameter. This formulation was able to overcome the key challenges associated with intravenous peptide delivery, pharmacokinetics, and plasma stability. In xenograft mouse models, the anticancer peptide nanoparticle was able to completely eradicate otherwise untreatable highly aggressive invasive human breast cancer.
A combination of membrane-active peptide and nano-formulation presents a vast pharmacological reservoir for the development of highly effective targeted anticancer treatments with low peripheral toxicity. This work demonstrates that seamless integration of multiple emerging techniques, ranging from 2D and 3D cell culture models, atomic detail molecular simulations, and nanotechnology enables development, tuning, characterization and demonstration of clinically relevant delivery of cancer-selective anticancer peptides that kill breast cancer cells at nontoxic levels, reveals a remarkable resilience against drug resistance formation in cancer stem cells, and can inhibit growth or eradicate human triple-negative breast cancer xenografts in mice. Building on an enormous body of work in the field of anticancer peptides, this study shows that clinical translation of membrane-active peptides may finally be within reach.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about ScienceX Dialog and how to participate.
More information:
Charles H. Chen et al, Integrated Design of a Membrane‐Lytic Peptide‐Based Intravenous Nanotherapeutic Suppresses Triple‐Negative Breast Cancer, Advanced Science (2022). DOI: 10.1002/advs.202105506
Bio: Charles H. Chen is a Senior Scientist in the Advanced Drug Delivery at AstraZeneca Pharmaceuticals. He got his Ph.D. degree in Chemistry at King's College London and has ten-year research experience in drug development and peptide therapeutics. Prior to joining AstraZeneca, he did 2-year postdoctoral research at Massachusetts Institute of Technology and Massachusetts General Hospital, where he worked on various peptide design approaches and studied bacteriophages and zebrafish models.