Activating cancer drugs by ultrasound signals
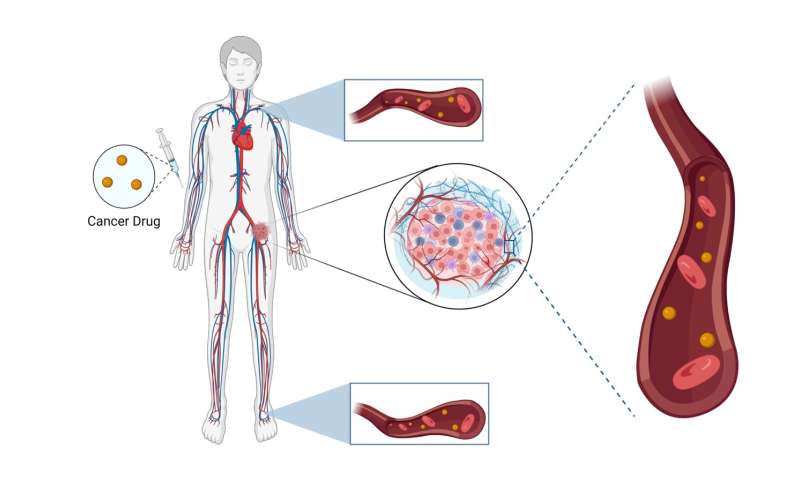
Have you ever thought about what makes cancer so tough to treat? Why are people still terrified when they are told they have "cancer" or a "tumor"? This question does not have a single answer since many factors contribute to the complexity of this disease.
Cancer is like a prodigal son to the body. A tumor is a group of fast-growing cells that wreaks havoc with the patient's health. This tumor gets bigger and fatter and steals the body's nutrients. If left untreated, this insatiable mass can attack nearby tissues, take over the whole body and kill it. The first problem arises when the body does not see cancer as a threat, but as a part of itself. When you attempt to kill cancerous tissues, you are, in fact, fighting the body. The patient's body will protect the cancerous tissue with all its power, something it has evolved to do over millions of years. So when treating cancer, doctors must circumvent sophisticated biological functions for protection.
Another major challenge is the nature of cancer therapy drugs. These drugs are designed to destroy cells indiscriminately, meaning they are not specific. They are poisons that are used for therapeutic applications. Imagine you have a cancer drug, and you want to administer it to a cancer patient. Almost all administration routes utilize the bloodstream. The bloodstream covers, literally, the whole body. So, your drug will enter a never-ending circulation cycle and reach every tissue in the body. Obviously, if drugs are poisons, the patient will experience serious detrimental side effects.
Therapy solutions
What are the solutions for these problems? Medical research has had significant success in reducing the toxicity of cancer drugs. Scientists have engineered drug carriers to cage toxic therapeutic agents and to escape the immune system. Caged drugs cannot be active while in the bloodstream. This is a fantastic accomplishment; however, it gives rise to another problem. In the caged form, drugs cannot be active when they reach tumor tissue, either. Furthermore, we have no sense of how much of the drug is released and delivered to the tumor tissue. This makes targeted drug uncaging in tumors critical. Controlled drug uncaging only inside the tumor can lead to tumor eradication while sparing healthy tissues. But uncaging drugs is not straightforward. Remember that the drug carriers are designed to keep drugs encapsulated.
Sound, a familiar tool
We produce audible sound waves in the throat with our vocal cords and make them meaningful by changing the structure of our lips and tongue. On the other hand, ultrasound consists of sound waves with inaudibly high frequencies. Ultrasound is used for fetal clinical imaging; however, the idea of our lab at the University of Texas at Dallas is to use ultrasound beyond this traditional application. Ultrasound can be used as an external energy source for drug uncaging. Our idea for triggering drug release is to sensitize conventional drug vehicles to ultrasound energy. This can be easily achieved by incorporating an ultrasound-sensitive agent in the drug carrier.
To uncage drugs from previously engineered drug vehicles, I and my Ph.D. advisor Dr. Shashank Sirsi have conjugated ultrasound-sensitive particles to conventional drug carriers and further demonstrated successful drug uncaging. We have published some of the associated research in the journal Pharmaceutics.
Ultrasound, a communication platform for drugs
Ultrasound-sensitive particles are simple in structure but complicated in behavior. These particles are responsive to ultrasound in the sense that they will change their shape upon ultrasound excitation. This shape change generates a secondary ultrasound wave which can be processed and quantified. The ultrasound-sensitive agent I used for drug uncaging is called a phase-changeable droplet. These droplets are spherical particles with a fluorocarbon liquid core. When in an ultrasound field, the liquid core will rapidly vaporize and produces a high amount of energy. Conventional drug carriers are destabilized by this fast phase-change event and drugs get uncaged. It is worth noting that vaporization is the same phenomenon behind the steam engine, the invention that changed the course of history.
This vaporization event also produces a secondary ultrasound signal that can be measured by clinical ultrasound settings. Vaporization can be controlled by ultrasound activation energy and monitored using the vaporization signal. So, you can imagine that by conjugating phase-changeable droplets to a drug carrier, we provide a platform to control drug release from the carriers. This is achieved by changing activation sounds and monitoring drug release by listening for the feedback sounds. It is as if you can change your tone, listen for a response, and fix your tone accordingly. As if you are engaging in a conversation.
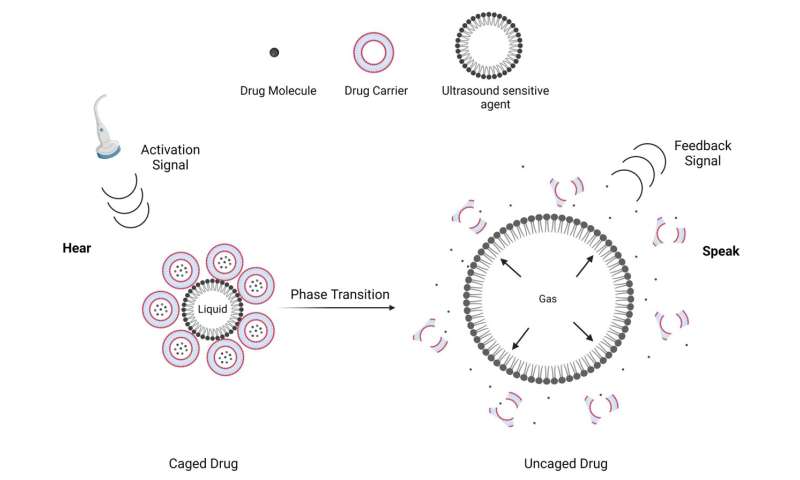
There is a need in the clinics to measure drug dosing in cancer patients. We hope that this research leads to designing pharmaceutical platforms where drug delivery can be accurately monitored and quantified.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about ScienceX Dialog and how to participate.
More information:
Arvin Honari et al, Improving Release of Liposome-Encapsulated Drugs with Focused Ultrasound and Vaporizable Droplet-Liposome Nanoclusters, Pharmaceutics (2021). DOI: 10.3390/pharmaceutics13050609
Arvin Honari is a fifth-year Ph.D. student at the University of Texas at Dallas. He is currently studying biomedical engineering where his research focuses on ultrasound-mediated drug delivery. His thesis focuses on designing ultrasound-sensitive drug vehicles for treating neuroblastoma, a rare pediatric malignancy that causes 15% of childhood cancer-related mortality.