Two-dimensional alkaline-earth hydroxides for microelectronics and carbon capture
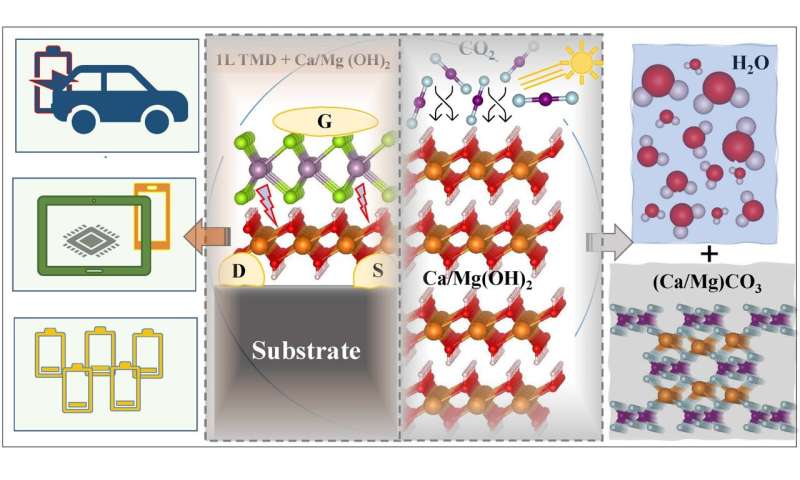
The design of microelectronic devices with two-dimensional (2D) materials is becoming more popular. But how interesting would it be to discover and use a new generation of multifunctional 2D materials for carbon capture and global warming? The multifunctionality of 2D materials means they have multifaceted characteristics that enable technologies to manipulate 2D structures for the advanced applications that nobody has ever envisioned.
Carbon capture and water splitting
In the past few years, theoretical simulation, artificial intelligence (AI) and experimental approach have come together to identify a wider range of novel 2D materials. Among the most recently studied 2D materials, alkali-earth hydroxides, such as Mg(OH)2 and Ca(OH)2 would help to protect our planet from the catastrophic effects of climate change. Although alkali-earth hydroxides are stable against oxidation at room temperature, they do not show stability against carbon dioxide (CO2). The instability of this class of 2D materials against CO2 enables them to contribute to the worldwide goal of decarbonization through carbon capture and heat storage.
In addition, because of their enhanced photocatalytic characteristics, 2D alkaline-earth hydroxides have significant benefits for photocatalytic water splitting. When compared to bulk (3D) photocatalysts, 2D photocatalysts are predicted to have appealing properties such as large specific surface areas accessible for photocatalytic reactions when exposed to visible light.
Water sensitivity and temperature dependency
Solubility is one of the critical factors in designing cutting-edge technologies with 2D materials. Since water can adversely affect the durability and performance of any device, less solubility in water is most desirable for technological applications of 2D alkaline-earth hydroxides. Interestingly, at room temperature, Mg(OH)2 is sparingly soluble in water and has a solubility very close to the solubility of amorphous SiO2.
Owing to the positive heat of the solution, the solubility of alkaline-earth hydroxides declines with increasing temperature making them desirable for future industrial applications, especially when a higher range of temperature is vital for the device operation. However, due to a high level of reactivity of Ca(OH)2 with water a true encapsulation architecture is mandated to improve its long-term stability when utilized for Ca(OH)2-based devices.
An alternative for 3D dielectrics
My colleagues and I at the University of Texas at Dallas (UTD) created a methodology to screen promising dielectrics for semiconductor industries. To design ultra-thin viable transistors, we demand a dielectric with a small thickness, a high dielectric constant, and a low leakage current. We calculated the leakage current of monolayer (1L) and bilayer (2L) alkaline-earth hydroxides to evaluate their performance as potential gate dielectric materials. We also modeled two heterostructures Ca(OH)2|HfS2 and Mg(OH)2|WS2 to assess the pertinence of alkaline-earth hydroxides when combined with 2D channel materials and proved that Mg(OH)2|WS2 heterostructure would be an effective structure in designing p-MOS and/or n-MOS transistors. However, other possible combinations require careful exploration to discover the most capable heterostructure candidates for future nano and microelectronics devices.
We hope that this research—published in Nanomaterials—will spur further investigation into alkaline-earth hydroxide materials for their applications in ultra-thin 2D field-effect transistors (FETs), carbon capture, and water splitting.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about ScienceX Dialog and how to participate.
More information:
Mehrdad Rostami Osanloo et al, A First-Principles Study on the Electronic, Thermodynamic, and Dielectric Properties of Monolayer Ca(OH)2 and Mg(OH)2, Nanomaterials (2022), DOI: 10.3390/nano12101774.
Mehrdad Rostami Osanloo has a Ph.D. in computational material physics from the University of Texas at Dallas. He is currently a Research Scientist in the modeling & AI team at Tokyo Electron Technology Center America (TTCA). His research mainly focuses on the investigation of electronic, dielectric properties of novel 2D materials and 2D defective structures. He employs theoretical and computational modeling to simulate and model the behavior of complex systems for designing the new generation of advanced materials that can ease the fabrication process of ultra-thin transistors used in the semiconductor industry.